Retroviral packaging is a process in which a retrovirus is used as a vector to efficiently package exogenous genes into viral particles to form infectious viral particles. This process plays a crucial role in the development process of gene therapy agents. CD Formulation relies on an advanced technology platform and an experienced team of experts, aiming to provide reliable retroviral packaging solutions. By utilizing retroviral packaging technology, we can help researchers achieve long-term stable gene expression by delivering therapeutic genes into host cells, and advance the gene therapy formulation process.
The Importance of Retroviral Packaging
- Efficient gene delivery. Retroviruses are able to efficiently transmit exogenous genes to host cells, including ones that do not divide, something that many other forms of viral vectors are unable to do.
- Gene integration. Long-term therapy may be possible because retroviruses can integrate the exogenous genes they carry into the host cell's genome. Once integrated, these genes will be expressed by all of the host cell's progeny.
- Safety. Replication-defective retroviral vectors, which are safer to employ since they cannot replicate themselves, can be created by removing specific genes from the virus.
- Targeting. Modifying retroviruses to target particular cell types is crucial for treating disorders in particular tissues or organs.
Retroviral packaging technology is of multifaceted importance in the process development of gene therapy products, which not only improves the efficiency and stability of gene transfer, but also helps to realize the large-scale production and research applications of gene therapy products.
Explore Our Retroviral Packaging
Vector construction
Construct a retroviral expression vector that contains the target gene as well as the necessary viral cis-acting elements.
Production of packaging plasmids
Preparation of a packaging plasmid containing genes for viral structural proteins that are required to form the outer shell of the viral particle. If infection of a specific cell type is desired, an envelope plasmid encoding the capsid protein is also included.
Cultivation of packaging cells
Co-transfect the packaging plasmid into packaging cells that are easily transfected and capable of producing large numbers of virus particles.
Transfection and viral particle production
Co-transfection of vector DNA into packaging cells using appropriate transfection methods such as electroporation, liposome-mediated transfection, etc. The cells will read these plasmids and start producing virus particles.
Collection of virus particles
Some time after transfection, the cells will release retroviral particles containing the target genes into the culture medium, and we need to collect the culture supernatant to obtain the virus particles.
Virus concentration and purification
The collected viral supernatant usually contains a large amount of cellular debris and protein impurities, which need to be concentrated and purified by ultracentrifugation, desalination filtration or other purification methods.
Viral titer assay
Specific methods, such as real-time PCR quantification of viral RNA, are required to determine viral titers to ensure efficient and consistent viral concentrations are used in subsequent experiments.
Viral efficiency and specificity validation
The function of the viral vector is confirmed by infecting target cells and examining their transduction efficiency and target gene expression. If designed to specifically infect a particular cell type, the specificity is also verified.
Safety evaluation
For subsequent applications, we also need to conduct adequate biosafety evaluation of retroviral particles, including testing of viral replication capacity and pathogen safety assessment.
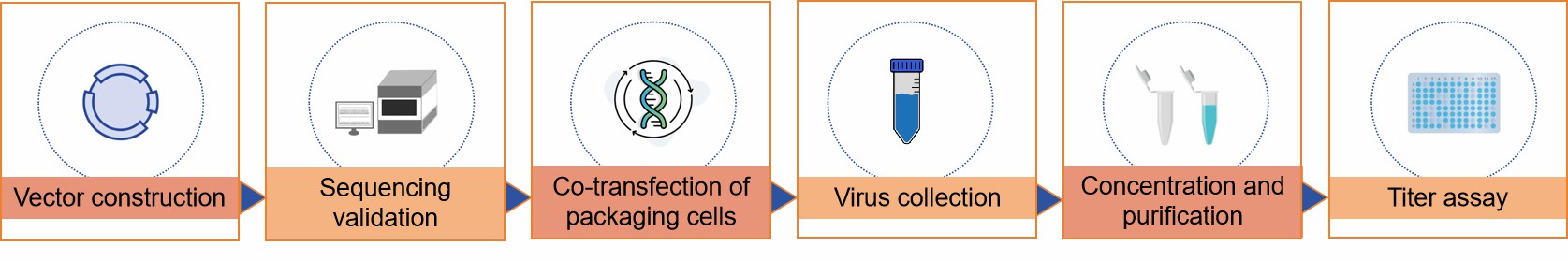 Fig.1 Retroviral packaging process. (CD Formulation)
Fig.1 Retroviral packaging process. (CD Formulation)
Our Platforms for Retroviral Packaging
| Platforms & Technologies |
Content Description |
| Retroviral vectors |
Retroviral vectors include different types such as MMLV, MSCV, etc., which are suitable for different types of cells and experimental needs. We can choose different vectors according to different experimental conditions and purposes. |
| Virus concentration technology |
We can concentrate and purify virus particles by PEG concentration, sucrose centrifugation and other methods to improve the efficiency of virus use. |
| Virus titer assay |
The titer determination of retroviruses is an important step to assess the efficiency of virus packaging, we often utilize qRT-PCR and other methods to quickly and accurately determine the viral titer. |
Highlights of Our Retroviral Packaging
- Flexibility. We can provide various envelope and carrier options to suit different experimental needs according to customer project characteristics.
- Strict quality control. We provide perfect quality control services, including viral titer determination, microbial detection, mycoplasma detection and so on.
- Customized service. We can provide customized viral packaging services according to the specific needs of our customers to meet their needs in the development of gene therapy agents.
- Technical support. We provide complete technical services and solutions, including providing detailed construct reports and professional technical support.
Published Data
Technology: Retroviral packaging
Journal: Ann N Y Acad Sci
IF: 5.2
Published: 1990
The article discusses the development of two packaging cell lines, GP + E-86 (ecotropic) and GP + envAm12 (amphotropic), for use in human gene therapy with retroviral vectors. These cell lines are designed to prevent the production of replication-competent viruses while generating high titers of replication-deficient vector viruses. The construction involves separating the viral gag and pol genes onto one plasmid and the env gene onto another, with deletions in the packaging sequence and 3' LTR to minimize risks. Tests showed no evidence of wild-type retrovirus production with these lines, and they effectively transferred the neoR gene into mouse hematopoietic cells, achieving successful long-term transplantation in irradiated recipients. Overall, the findings suggest that these packaging cell lines are both safe and efficient for gene therapy applications in both murine and human contexts.
CD Formulation retrovirus packaging and quality control adopts a standardized process, and the virus particles obtained are purified by ultracentrifugation and the virus titer is determined by qPCR, which can meet the requirements for various experiments. If you are interested in us, please feel free to contact us.
References
- Markowitz D, et al. Retroviral gene transfer using safe and efficient packaging cell lines. Ann N Y Acad Sci. 1990, 612:407-14.
Related Services


 Fig.1 Retroviral packaging process. (CD Formulation)
Fig.1 Retroviral packaging process. (CD Formulation) 