Adeno Associated Virus Packaging
Inquiry
Recombinant adeno-associated virus (AAV) is a widely used viral vector for both in vitro and in vivo experiments. AAV can transduce a large number of mammalian cell types and exhibits non-pathogenicity and low immunogenicity in the human body, characteristics that have made it one of the most efficient gene delivery vectors currently used in gene therapy applications. CD Formulation provides high-quality AAV packaging services to meet the experimental needs associated with AAV gene therapy. We have continuously enhanced our adeno-associated virus packaging process to significantly improve the titer, purity, activity, and consistency of our AAV.
Technical Advantages of AAV Packaging
- High safety. So far, wild-type AAV has never been found to be pathogenic to humans, recombinant AAV is a three-plasmid system, the genome sequence removes most of the wild-type AAV genome elements, only the ITR sequence and other genes are expressed by independent plasmids, which further ensures the safety.
- Low immunogenicity. The genome of AAV2 has only 4681 nucleotides, which is easy to operate with conventional recombinant DNA technology, and the immune response caused by animal experiments is small, and AAV will rarely be cleared by the immune system after infecting tissues.
- Wide host range. AAV can infect a wide range of mammals has been successfully applied to the expression of human and non-human proteins, and can transfect not only dividing cells but also non-dividing cells.
- Multiple serotypes. AAV1 to AAV9 and 12 serotypes such as DJ, DJ/8, Rh10, etc. Different serotypes of AAV can target different cells and tissues.
- Stable expression and long duration. Not integrated into the host genome, can be a long-term stable expression of exogenous genes, in the host cell to form an add-on (episome) that exists in the cell nucleus, in vivo can be sustained expression for more than 6 months.
- Highly diffusible. In contrast to lentiviruses and adenoviruses, AAV can penetrate the blood-brain barrier and is the most suitable for infecting neurons and glial cells.
Process for AAV Packaging
AAV is a small, non-pathogenic virus with a single-stranded DNA genome. The packaging process of AAV is divided into the following steps.
Construction of AAV plasmid
The packaging of AAV begins with the construction of an AAV plasmid containing the desired genes. This plasmid usually includes the two terminal inverted repeats (ITRs) of the AAV genome, as well as the gene sequence to be delivered. Appropriate regulatory sequences, such as promoters and terminators, are also usually included in the construction of the plasmid to ensure proper gene expression.
Transfection of cells
The constructed AAV plasmid is transfected into packaging cells. Commonly used packaging cells include the HEK293 cell line, which is highly efficient for AAV replication and packaging.
Extraction of AAV particles
After transfection, the AAV genome and packaging proteins are assembled into complete AAV particles in packaging cells. Subsequently, pure AAV particles can be extracted from the cells through cell rupture and purification steps.
Quality control assay
The extracted AAV particles need to be subjected to quality control assays to ensure that their quality meets the requirements. Commonly used assays include PCR, immunoblotting, and electron microscopy observation, which can verify the integrity, purity, and activity of AAV particles.
Obtaining purified AAV
After quality control testing, highly pure and active AAV particles can be obtained for further gene delivery studies or clinical applications.
 Fig.1 AAV packaging process. (CD Formulation)
Fig.1 AAV packaging process. (CD Formulation)
Applications of Our AAV Packaging
- Gene therapy. AAV is widely used in gene therapy for the treatment of various hereditary diseases, cancers, and neurological disorders. By loading therapeutic genes into AAV particles for delivery, the precise delivery and expression of genes can be realized, to repair or replace damaged genes and achieve therapeutic purposes.
- Biomedical research. AAV packaging technology is also widely used in biomedical research. Researchers can use AAV particles to introduce gene sequences of interest into cells or animal models for the study of gene function, disease mechanisms, and other issues.
- Gene transduction tools. In addition to gene therapy and research, AAV packaging technology is also used as a gene transduction tool. Researchers can use AAV particles to introduce fluorescently labeled genes, reporter genes, etc. into target cells for applications such as tracking cell fate and labeling specific cell types.
Information for Customers
- If we are commissioned to construct the vector, the customer needs to provide the contract number and gene name of the vector construction, titer, total amount requirement, and serotype.
- If the customer provides the vector, they need to provide the vector sequence file, or the location of the enzyme cut site and the sequence length between the AAV ITR.
Our Platforms for AAV Packaging
| Platforms & Technologies |
Content Description |
| Baculovirus-AAV system |
This is a method for AAV production in insect cells using baculoviruses that produces high-titer AAV particles suitable for ultra-purified AAV production at the in vivo application level. |
| Diversification of AAV serotypes |
A variety of AAV serotypes have been developed, such as AAV1 to AAV9 as well as DJ, DJ/8, PHP.eB, PHP.S, etc. Each serotype has different hydrophilicity and can be targeted and selected for infection of specific cell or tissue types. |
| Ultra-purification technology |
AAV particles are further purified and concentrated by methods such as CsCl gradient density centrifugation to provide a highly pure AAV product suitable for a wider range of research and therapeutic applications. |
Highlights of Our AAV Packaging
- We provide our customers with high purity and high titer AAV particles by optimizing the AAV packaging process.
- We can provide customized AAV vector design and packaging services according to different research needs to meet customers' different project requirements.
- We provide professional technical support and question-answering services to help customers solve various problems encountered during the AAV packaging process.
- We can produce AAV on a large scale, which can meet the needs of different scales from laboratory research to clinical applications.
Published Data
Technology: Recombinant adeno-associated viruses (rAAVs) packaging
Journal: J Virol
IF: 4.8
Published: 2021
The novel Rep hybrids from different AAV serotypes described in this study were able to reduce the percentage of empty capsids in all AAV serotypes tested while increasing the overall yield of genome-containing AAV capsids. They can likely be easily integrated into existing AAV manufacturing protocols to optimize the production of the generated AAV gene therapy products.
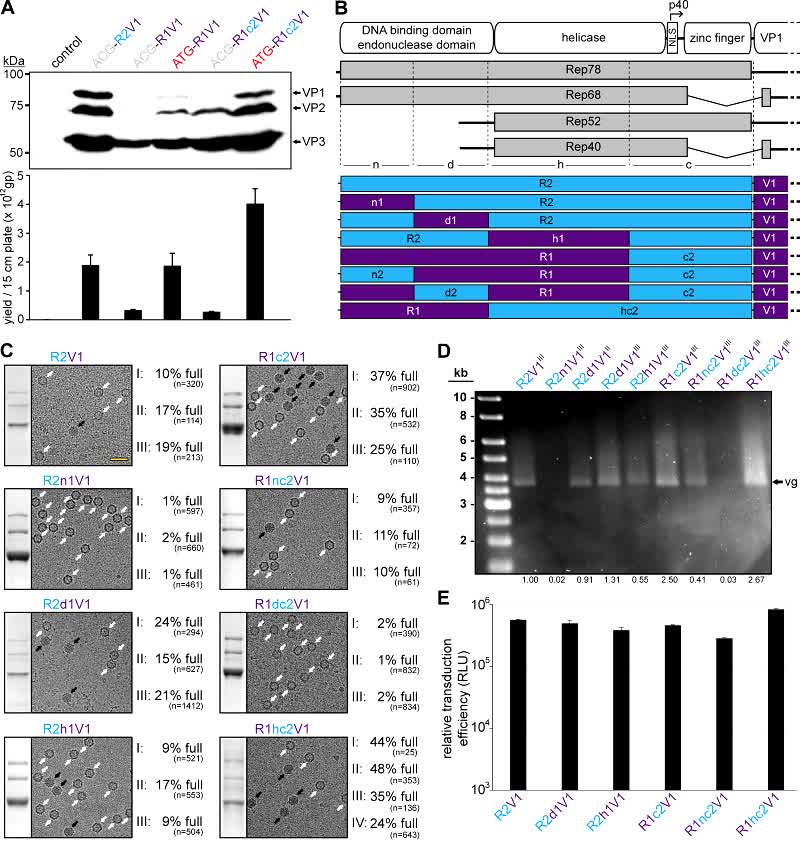 Fig.2 AAV1 packaging efficiency. (Mietzsch M, et al., 2021)
Fig.2 AAV1 packaging efficiency. (Mietzsch M, et al., 2021)
CD Formulation offers AAV packaging services that show promise in scientific research animal and cellular experiments. We offer a wide range of AAV customized titers for your various experimental needs. Through our innovative AAV packaging process, we aim to provide our customers with AAV with high purity and high titer. If you are interested in us, please feel free to contact us.
References
- Mietzsch M, et al. Improved Genome Packaging Efficiency of Adeno-associated Virus Vectors Using Rep Hybrids. J Virol. 2021 Sep 9;95(19):e0077321.
Related Services

 Fig.1 AAV packaging process. (CD Formulation)
Fig.1 AAV packaging process. (CD Formulation) Fig.2 AAV1 packaging efficiency. (Mietzsch M, et al., 2021)
Fig.2 AAV1 packaging efficiency. (Mietzsch M, et al., 2021)