Adenovirus is a common class of viruses used as tools in gene delivery and gene therapy research. Adenovirus packaging is the process of loading the gene sequence of interest into an adenovirus particle. Adenovirus has become one of the most promising viral vectors in the field of gene therapy. CD Formulation provides adenoviral packaging services that can be used to significantly enhance the adenoviral packaging process in terms of viral titer, purity, activity, and consistency.
Technical Advantages of Adenovirus Packaging
- High infection efficiency. The viral titer can be as high as 1011 PFU/mL, which can be effectively proliferated and is suitable for gene therapy.
- Wide host range. Commonly used in the infection of difficult-to-infect cells, not only can be infected with ordinary cell lines but also can be infected with suspension cells, primary cells, etc.
- Fast expression. After infecting cells, it can be expressed in 1 to 2 days, which is the best choice for studying gene expression in primary cells and suspension cells.
- Low pathogenicity and high safety. The genome carried by adenovirus is not integrated into the chromosome of the host cell and does not interfere with other host genes.
Process for Adenovirus Packaging
Vector construction
We first need to construct an adenoviral vector that contains a large portion of the adenoviral genome with the gene sequence of interest inserted in a specific locus.
Transfection of cells
We introduce the constructed adenoviral vector into host cells, a common cell line used is 293 cells.
Overexpression of genes
The inserted gene sequence is transcribed and translated into the host cell to produce the corresponding protein. This step can be used to express a gene of interest or for gene therapy.
Collection of virus particles
Adenovirus particles produced in the host cell are released into the culture supernatant by lysis or other means, and the supernatant is then collected.
Purification and concentration
We purify and concentrate the supernatant using centrifugation and filtration to obtain adenovirus particles of high purity and concentration.
The specific process and conditions of adenovirus packaging will be optimized and modified to some extent according to the experimental settings and research objectives.
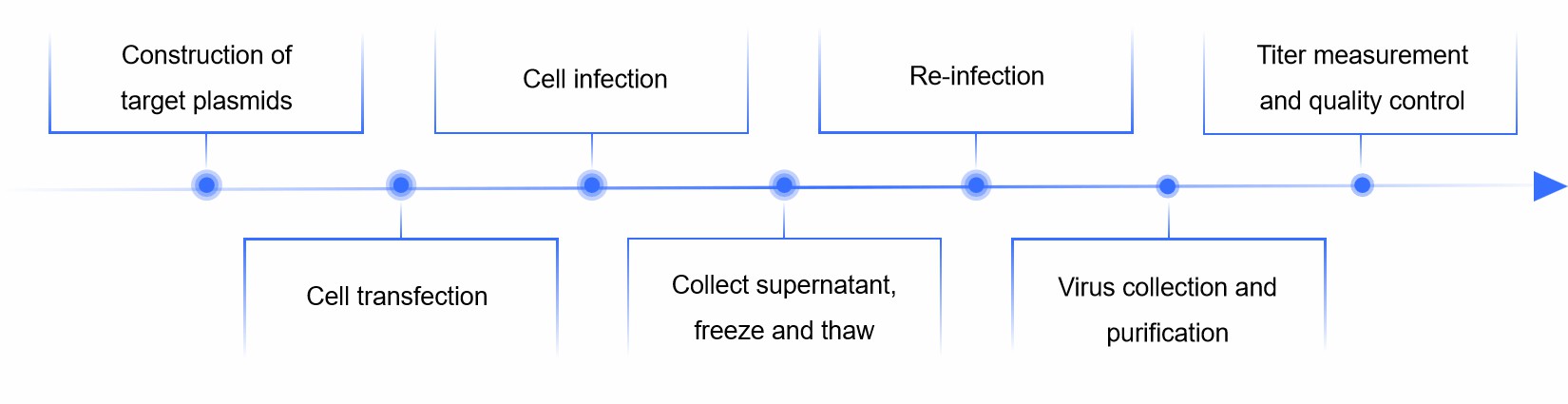 Fig.1 Adenovirus packaging process. (CD Formulation)
Fig.1 Adenovirus packaging process. (CD Formulation)
The adenovirus vectors we provide are effective in infecting host cells and can meet the research needs of many researchers. The recombinant adenovirus system we use contains an EGFP reporter gene, and the recombinant packaged adenovirus can be observed under a fluorescence microscope to determine the degree of cell infection after infecting the target cells according to the experimental requirements.
Our Platforms for Adenovirus Packaging
| Platforms & Technologies |
Content Description |
| Optimized plasmid design |
With the development of molecular biology technology, it has become a hot research topic for us to continuously optimize plasmid design to improve the efficiency of adenovirus packaging. For example, better promoters, enhancers, and self-splicing sequences are used to improve the expression of target genes. |
| Improved viral vectors |
We offer a new generation of adenoviral vectors that show higher infection efficiency and cell specificity, which is expected to improve the limitations of previous vectors in some applications. |
| Targeted modification technology |
We achieve cell or tissue specificity of adenoviruses through surface modification or genetic modification. For example, screening and binding of tissue-specific ligands using peptide library technology has improved adenovirus targeting ability. |
Highlights of Our Adenovirus Packaging
- Wide range of lentivirus, adenovirus, and adeno-associated virus packaging services.
- Cost-effective, get the same amount of virus less funding.
- Strict quality control, no other microbial contamination such as mycoplasma.
- Reliable quality, our cooperative customers have published many papers.
Published Data
Technology: Adenovirus packaging
Journal: J Virol
IF: 4.8
Published: 2003
In the case of adenovirus, sequences between nucleotides 200 and 400 at the left end of the genome are essential for packaging. This region contains a series of redundant bipartite sequences, termed A repeats, that function in packaging. Synthetic packaging sequences made of multimers of a single A repeat substitute for the authentic adenovirus packaging domain. A repeats are binding sites for the CCAAT displacement protein and the viral protein IVa2. Several lines of evidence implicate these proteins in the packaging process. The author utilized an in vivo approach to address the role of the inverted terminal repeats and the covalently linked terminal proteins in packaging of the adenovirus genome. This result indicates that these elements are not required for the viral genome to be packaged efficiently. These findings have important implications for the design of gene therapy vectors, one of which is that the adenovirus packing domain's attachment to heterologous DNA sequences should be sufficient to target the viral capsid.
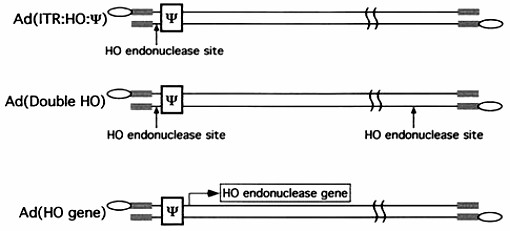 Fig.2 Schematic diagram of the structures of the adenovirus. (Ostapchuk P, et al., 2003)
Fig.2 Schematic diagram of the structures of the adenovirus. (Ostapchuk P, et al., 2003)
CD Formulation offers adenovirus packaging services that are engineered and optimized to greatly enhance biosafety. If you are interested in us, please feel free to contact us.
References
- Ostapchuk P, et al. Minimal cis-acting elements required for adenovirus genome packaging. J Virol. 2003, 77(9):5127-35.
Related Services

 Fig.1 Adenovirus packaging process. (CD Formulation)
Fig.1 Adenovirus packaging process. (CD Formulation) Fig.2 Schematic diagram of the structures of the adenovirus. (Ostapchuk P, et al., 2003)
Fig.2 Schematic diagram of the structures of the adenovirus. (Ostapchuk P, et al., 2003)