Gene Therapy Formulation Impurity Testing
Inquiry
Gene therapy impurity testing plays an important role in ensuring the importance of the development of gene therapy formulations, helping to ensure that they are safe, as well as meeting the requirements of regulatory agencies. CD Formulation offers a range of gene therapy formulation impurity testing suits for detecting mycoplasma and bacterial contamination, exogenous factors, abnormal toxicity, and other assay analyses. Our experts can develop a comprehensive impurity testing program to support the progress of your gene therapy formulation development.
Importance of Gene Therapy Formulation Impurity Testing
- Ensure efficacy. Testing gene therapy formulations for impurities, such as purity assessment and impurity control, can ensure the effectiveness of gene therapy formulations.
- Meet regulatory requirements. Rigorous impurity testing is necessary to meet the requirements of regulatory agencies, which helps to get the product approved and marketed.
- Optimize manufacturing processes. Impurity testing of gene therapy formulations can be used to guide improvements in the manufacturing process and further enhance product quality.

Explore Our Gene Therapy Formulation Impurity Testing
We provide comprehensive and accurate testing services for gene therapy formulation impurity testing. We aim to provide our clients with safer and more effective gene therapy formulations by testing for microbial contamination, exogenous factors, and genotoxic impurities. Our current testing services include but are not limited to, impurity testing, sterility testing, reverse transcriptase activity assay, exogenous factor testing, and abnormal toxicity testing. We will list the specific testing services below. To date, our impurity testing services have assisted drug developers in testing several gene therapy formulations with a high degree of accuracy. Below is a brief list and description of some of our impurity testing.
We provide process-related impurity testing for gene therapy formulations. As the production process often involves multiple steps such as cell culture, viral vector production, purification, etc., various impurities, such as host cell DNA, host cell proteins, and culture medium components, may be introduced. Process-related impurity testing is required to ensure that these impurities are controlled within reasonable limits and to minimize immune responses or other adverse effects on patients.
We offer sterility testing for gene therapy formulations to ensure that the product does not lead to serious infections and complications in subsequent applications. Our sterility testing includes the detection of bacteria, fungi, and mycoplasma, and testing methods include DNA staining, PCR, and others.
In addition, like the abnormal toxicity test for gene therapy formulations, through animal experiments, it detects whether the agents cause abnormal toxic reactions to help identify potentially toxic impurities, such as residual chemicals, degradation products, and so on.
Our Process of Gene Therapy Formulation Impurity Testing
The impurity detection of gene therapy formulations is a systematic, multi-step process involving comprehensive testing of raw materials, production processes, and finished products. Through strict impurity testing, the quality of gene therapy formulations can be effectively controlled to ensure their safety, efficacy, and quality controllability.
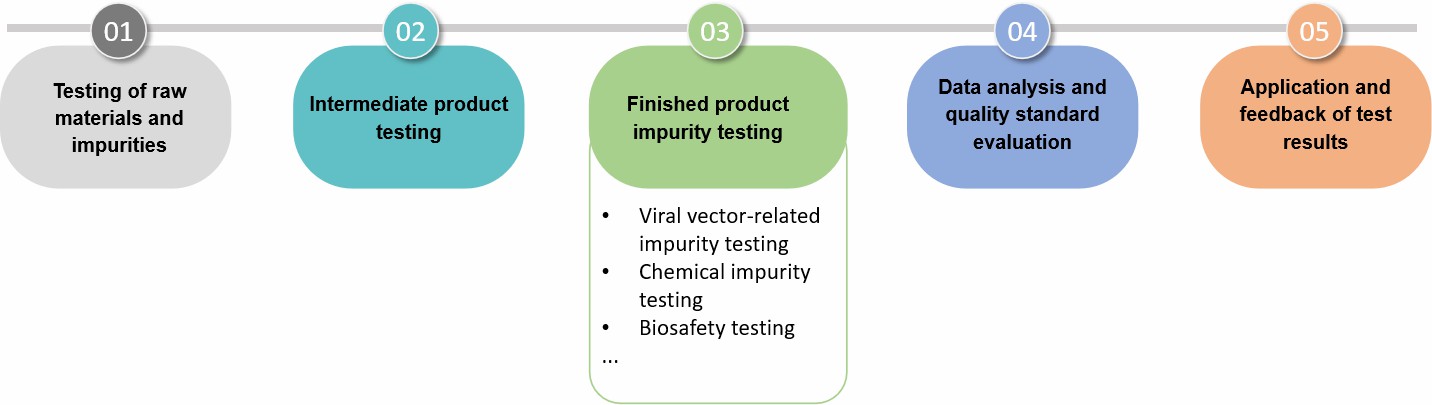 Fig.2 Our process of gene therapy formulation impurity testing. (CD Formulation)
Fig.2 Our process of gene therapy formulation impurity testing. (CD Formulation)
Our Platforms for Gene Therapy Formulation Impurity Testing
| Technologies & Platforms |
Content Description |
| Next generation sequencing (NGS) |
In gene therapy formulation development, NGS technology can quickly and accurately detect contaminants, impurities, mutations, and gene sequences in viral samples, providing comprehensive information to ensure product quality. |
| Digital PCR technology (ddPCR) |
Digital PCR technology has good application value in the detection of impurities in gene therapy agents. ddPCR achieves absolute quantitative detection of target nucleic acids by dispersing the sample into tens of thousands of nanoliter-volume microdroplets, each of which undergoes PCR amplification independently. We utilize this technology for the detection of residual DNA in host cells, detection of exogenous microbial contamination, etc. |
| High-performance liquid chromatography (HPLC) |
HPLC is effective in the detection of impurities in gene therapy preparations, such as detecting residual chemical impurities, detecting the purity of viral vectors, etc., and also separating and detecting a wide range of impurities in complex samples. |
Highlights of Our Gene Therapy Formulation Impurity Testing
- Scientific management system. We have constructed a digital quality management platform covering the whole process and adopted an advanced quality assessment system to track the whole process of gene therapy preparations.
- Expert support. Our expert team, an interdisciplinary team consisting of several senior researchers, covers specialized fields such as viral vector development, molecular biology, and pharmacokinetics.
- Customized service. We have designed a modularized service portfolio for the special needs of different R&D phases, and support dynamic adjustment of service content according to the progress of the project, so as to achieve the optimal allocation of R&D resources.
- State-of-the-art technology platform. Our laboratories are equipped with international leading equipment clusters such as high-resolution mass spectrometers, and we have set up an analysis system that includes six major detection dimensions, including host protein residues and process-related impurities.
As an industry leader in the field of gene therapy formulation development, CD Formulation has provided impurity analysis services for many gene therapy formulation development companies around the world. If you are interested in us, please feel free to contact us.
Related Services


 Fig.2 Our process of gene therapy formulation impurity testing. (CD Formulation)
Fig.2 Our process of gene therapy formulation impurity testing. (CD Formulation) 