Gene Therapy Formulation Abnormal Toxicity Assay
Inquiry
Abnormal toxicity testing is a systematic assay designed to assess the various toxic reactions that may result from gene therapy agents in vivo. This testing usually involves performing it in animal models, such as mice, rats, and rabbits. By observing the physiological and pathological changes in animals after receiving a biological product, the toxic potential of the biological product can be understood. This test helps to assess and recognize potential toxic reactions, thus ensuring that the use of biologics does not pose a risk to human health. CD Formulation relies on an advanced technology platform and years of experience in research services, we can provide reliable and accurate abnormal toxin test services to support our clients' research projects, to help you develop gene therapy formulations.
The Importance of Gene Therapy Formulation Abnormal Toxicity Assay
- Protecting patient safety. Abnormal toxicity testing helps assess the potential risks of gene therapy agents and ensures that patients are using safe products. Because there are many biological similarities between humans and animals, toxicity testing in animal models can help predict possible reactions in humans.
- Improve product quality. Abnormal toxicity testing can assess not only the potential toxicity of gene therapy formulations but also their efficacy and safety. Through systematic testing, the quality and efficacy of gene therapy preparations can be better improved.
- Regulatory requirements. Regulations in many countries and regions require abnormal toxicity testing of biologics, including gene therapy preparations. This is to protect the interests of patients and to ensure that the products comply with relevant regulations and standards.
Explore Our Gene Therapy Formulation Abnormal Toxicity Assay
There are various methods for abnormal toxicity testing, and we usually select the appropriate method based on the characteristics of the client's project, the characteristics of the gene therapy formulation, and the purpose of use. The following are the abnormal toxicity testing methods we commonly use.
Acute toxicity test
This test is used to identify the acute toxicity of gene therapy agents to tissues and organs. The test is usually conducted in an animal model to observe and record the physiological changes, mortality and side effects of the animals after the use of the biologics.
Subacute toxicity test
This test is designed to assess the subacute or subchronic toxicity response that may result from long-term exposure to a biologic. By giving the animals a certain dose of gene therapy formulation continuously, observe and record the physiological indexes, blood chemical indexes and pathological changes of the animals.
Skin irritation and allergenicity test
This test is used to assess the irritation and allergic reactions that may result from skin contact with a gene therapy formulation. The test usually involves applying the biological product to the skin of the animal and observing and recording any skin reactions.
Genotoxicity test
This test is used to assess the effect of a biological product on genetic material, such as gene mutations or chromosomal abnormalities. This test is usually performed in cell culture and assesses the genotoxicity of a gene therapy formulation by detecting chromosomal aberrations or gene mutations.
Our Process of Gene Therapy Formulation Abnormal Toxicity Assay
Preparation of test solution
Make the test solution according to the concentration specified under the species, and equilibrate it to room temperature before use.
Selection of test animals
Select healthy and qualified test animals, which should be kept under normal feeding conditions before the test and during the observation period, and the used animals should not be reused.
Method of drug delivery
Determine the mode of administration according to the characteristics of the formulation.
Observation and test
Observe the test animals after the administration of the drug, the specific observation objectives are determined by the experimental purpose. In addition, it is necessary to pay attention to the weight change of the test animals during the observation period.
Judgment of results
Based on the survival of the animals during the observation period, weight changes and whether there is any abnormal reaction to determine whether the test material complies with the regulations.
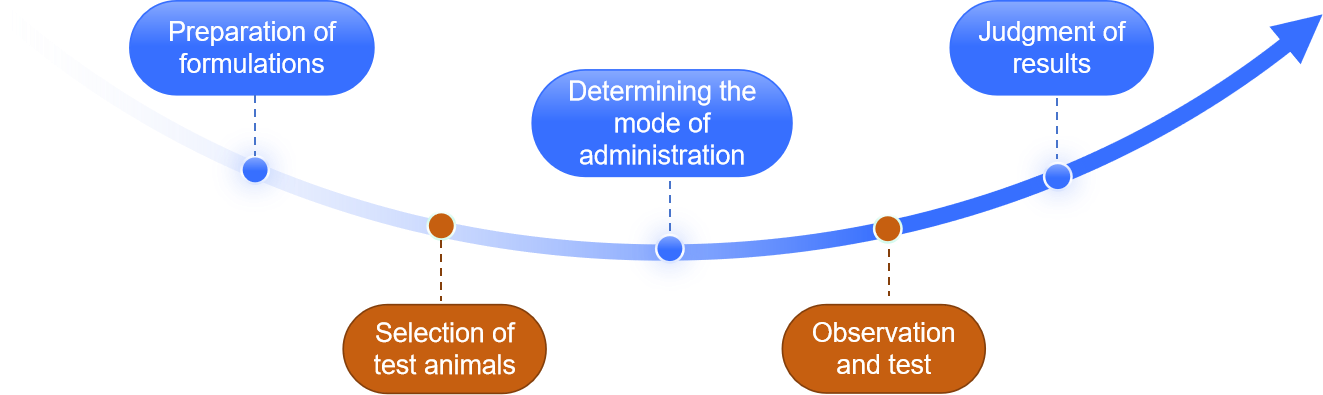 Fig.2 Our process of abnormal toxicity testing. (CD Formulation)
Fig.2 Our process of abnormal toxicity testing. (CD Formulation)
Our Technologies for Gene Therapy Formulation Abnormal Toxicity Assay
| Platforms & Technologies |
Content Description |
| Mouse abnormal toxicity test method |
This is an in vivo test method in which a certain dose of a test article solution is injected into mice or administered orally, and deaths occurring in the mice are observed within a specified period of time to determine whether the test article complies with the regulations. |
| Pyrogen check method |
The pyrogen check involves injecting the test material into the rabbit and is used to determine whether the limit value of pyrogens contained in the test material complies with the regulations by observing the increase in body temperature of the rabbit. This method has a long history, but is gradually being replaced by the bacterial endotoxin test. |
| Bacterial endotoxin check method |
This method utilizes specific reagents to detect or quantify bacterial endotoxins produced by gram-negative bacteria to determine if the limit of bacterial endotoxins in a test article is in compliance. |
Highlights of Our Gene Therapy Formulation Abnormal Toxicity Assay
- Diversification. We provide a wide range of services including testing, analysis, and development to ensure full support for your gene therapy formulation development program.
- Specialization. With a strong core technical team, we aim to provide high-quality services to meet the diverse and specialized needs of different customers.
- Compliance. We have built a corporate compliance management system to ensure scientific, standardized and orderly operation.
Published Data
Technology: Gene therapy immunogenicity and toxicity testing
Journal: Front Immunol
IF: 7.3
Published: 2023
Recombinant adeno-associated virus (AAV) as a vector for gene therapy is challenged by immune reactions and adverse effects despite showing great potential in preclinical and clinical studies. High doses of AAV vectors may lead to complement activation and hepatotoxicity, and even cause subject death in some clinical trials. Immunosuppressive agents, while controlling some adverse events, are not effective in all participants. This study describes the immunogenicity safety of AAV gene therapy, and further research and careful study design are needed in this area. At the same time, the potential of AAV for the delivery of biological therapies should not be overlooked, and the choice of the coat is critical for improving therapeutic efficacy.
Abnormal toxicity testing is essential to ensure the safety and quality of gene therapy formulations. By evaluating potential toxic reactions, problems can be detected and solved in advance to avoid harm to the human body. CD Formulation can help researchers perform the necessary abnormal toxicity testing for gene therapy formulations. If you are interested in us, please feel free to contact us.
References
- Bradbury A, et al. Editorial: Immunogenicity and toxicity of AAV gene therapy. Front Immunol. 2023 Jun, 14:1227231.
Related Services


 Fig.2 Our process of abnormal toxicity testing. (CD Formulation)
Fig.2 Our process of abnormal toxicity testing. (CD Formulation)