Gene Therapy Formulation Reverse Transcriptase Activity Assay
Inquiry
Gene therapy formulations need to be examined for reverse transcriptase activity during production and validation, which is used to ensure the safety of gene therapy products. Reverse transcriptase activity assay is a commonly used test that can indicate the possible presence of retroviruses. The detection of reverse transcriptase activity can indirectly reflect the presence of viral contamination in biologics, providing an important basis for the monitoring of the production process and regulatory requirements. CD Formulation has many years of experience in the development and testing of gene therapy formulations. We provide comprehensive and professional reverse transcriptase activity testing services to support the development of gene therapy formulations.
The Importance of Gene Therapy Formulation Reverse Transcriptase Activity Assay
- Ensure product safety. In gene therapy formulations, any viral contamination can lead to serious safety concerns. Reverse transcriptase activity testing can be used for early detection of potential viral contamination so that appropriate measures can be taken to ensure the safety of the final product.
- Meet regulatory requirements. Regulatory agencies have strict requirements for quality control of biologics. Reverse transcriptase activity testing, as a standardized test, ensures that gene therapy formulations meet the requirements of the relevant regulations and reduce the risk of compliance.
- Improve production efficiency. Through regular testing of reverse transcriptase activity, problems can be detected in the production of gene therapy formulations promptly, thereby improving production efficiency and reducing costs.
Explore Our Gene Therapy Formulation Reverse Transcriptase Activity Assay
Sample preparation
Cell culture supernatant is collected, centrifuged to remove cellular debris, filtered and subjected to ultracentrifugation to collect viral particles. Viral particles are resuspended using specific sample dilutions to prepare samples for testing.
Reverse transcriptase dilution
Gradient dilutions are performed using known concentrations of reverse transcriptase standards to establish a standard curve.
Reverse transcription reaction (RT)
The sample to be tested is mixed with reverse transcriptase, primers, probes and reaction buffer and the reverse transcription reaction is performed at a specific temperature to convert the RNA template to cDNA.
Real-time fluorescent quantitative PCR (qPCR)
Using the reverse transcription product as a template, qPCR amplification is performed to detect and quantify reverse transcriptase activity by the increase in fluorescence signal.
Data processing
Calculate the concentration of reverse transcriptase in the sample according to the standard curve, and judge whether reverse transcriptase activity is present in the sample by the Ct value.
Judgment of results
Based on the experimental results, the presence or absence of reverse transcriptase activity in the gene therapy formulation is judged, thus assessing whether the formulation may contain retroviruses.
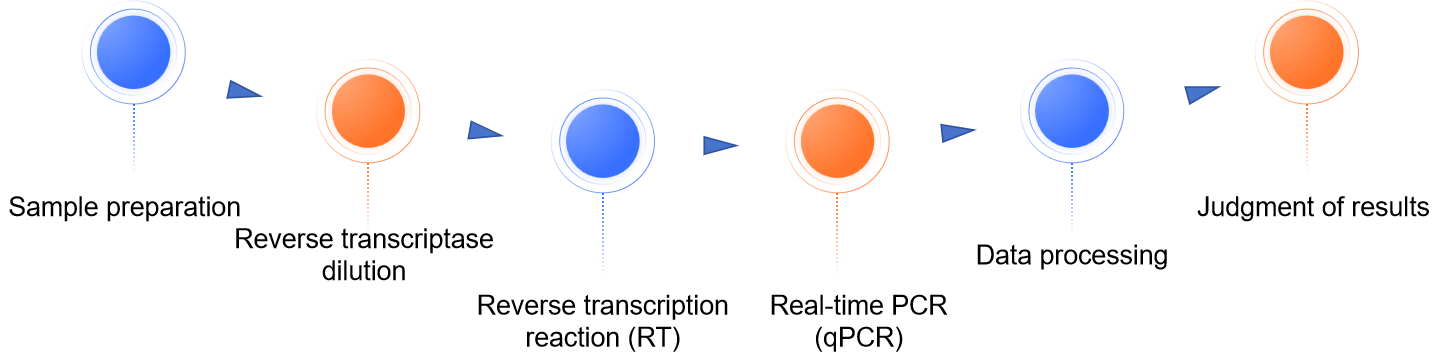 Fig.1 Our process of reverse transcriptase activity assay. (CD Formulation)
Fig.1 Our process of reverse transcriptase activity assay. (CD Formulation)
Our Technologies for Gene Therapy Formulation Reverse Transcriptase Activity Assay
| Platforms & Technologies |
Content Description |
| Quantitative PCR (qPCR) |
This method quantifies reverse transcriptase activity by monitoring the fluorescence signal changes in the PCR reaction in real time. It has the advantage of high sensitivity and specificity, and is suitable for the detection of low concentrations of RNA samples. |
| Enzyme-linked immunosorbent assay (ELISA) |
ELISA can be used to detect reverse transcriptase activity by binding to a specific antibody to form a complex, which can then be quantified by spectroscopic or fluorescent signals. |
| Colorimetric assay |
This method utilizes the product produced by the reverse transcriptase-catalyzed reaction, the concentration of which is determined colorimetrically. It is easy to operate and suitable for rapid screening. |
| Reverse transcriptase activity ratio method |
The activity of reverse transcriptase in a sample is compared with a standard enzyme of known concentration, and the ratio is calculated to determine the activity of reverse transcriptase in the sample. |
Highlights of Our Gene Therapy Formulation Reverse Transcriptase Activity Assay
- Advanced testing technology. We have an international leading detection technology platform to ensure the accuracy of reverse transcriptase activity detection.
- Professional technical team. We have an experienced team of experts and technicians to ensure that we provide professional technical support and effective solutions to our customers.
- Customized service. We can provide customized reverse transcriptase activity test solutions according to the specific needs of customers, to maximize the satisfaction of customer requirements.
- Strict quality control. We strictly follow the corresponding standard operation procedures and quality control measures when testing reverse transcriptase activity to ensure the reliability of the test results.
Published Data
Technology: Reverse transcriptase activity assay
Journal: Cell Mol Life Sci
IF: 6.2
Published: 2010
Reverse transcription is essential for retroviruses and retrotransposons, carried out by reverse transcriptase (RT), which converts viral RNA into double-stranded DNA for integration. While all RTs share similar functions, they vary in their catalytic properties, structures, and subunit composition. HIV-1 RT, critical in AIDS treatment, is a primary target for antiretroviral drugs. Most research focuses on HIV-1 RT, though other RTs have contributed to the broader understanding of retrovirology. This review covers RT properties, including their structure, functions, interactions, and resistance to antiretroviral therapies.
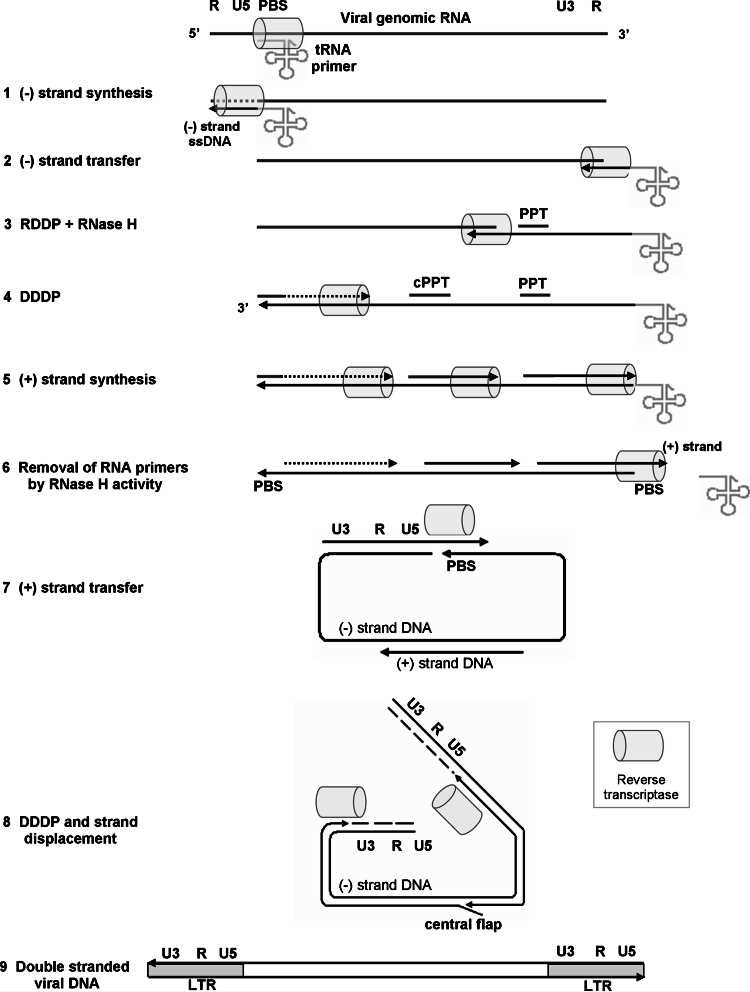 Fig.2 The reverse transcription process. (Herschhorn A, et al., 2010)
Fig.2 The reverse transcription process. (Herschhorn A, et al., 2010)
CD Formulation has a state-of-the-art reverse transcriptase biopsy platform for the early detection of viral contamination through testing of gene therapy formulations for process control and regulatory compliance. If you are interested in us, please feel free to contact us.
References
- Herschhorn A, et al. Retroviral reverse transcriptases. Cell Mol Life Sci. 2010, 67(16):2717-47.
Related Services

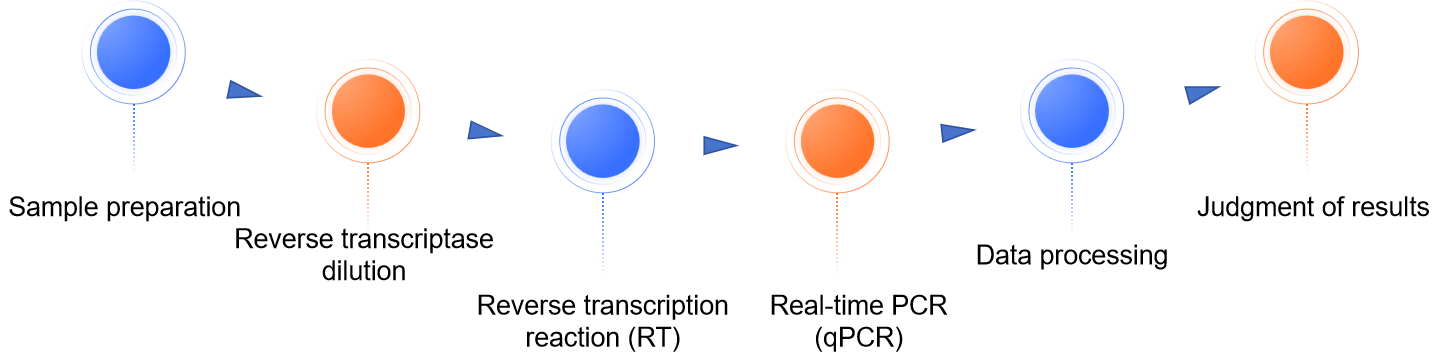 Fig.1 Our process of reverse transcriptase activity assay. (CD Formulation)
Fig.1 Our process of reverse transcriptase activity assay. (CD Formulation) Fig.2 The reverse transcription process. (Herschhorn A, et al., 2010)
Fig.2 The reverse transcription process. (Herschhorn A, et al., 2010)