Viral vectors are important tools used in gene therapy to deliver therapeutic genes into host cells, and testing of viral vectors is critical to ensure the safety, efficacy, and quality of gene therapy formulation development. CD Formulation provides accurate and reliable viral vector testing services for quality control of gene therapy formulations upon request to ensure the safety and efficacy of gene therapy formulations and help researchers facilitate gene therapy formulations' development.
The Importance of Viral Vector Testing
- Safety assessment. The safety of gene therapy is assessed by testing the purity and integrity of the viral vectors to ensure that they do not contain impurities or mutant viruses that may cause abnormal reactions in host cells.
- Efficacy validation. To assess the effectiveness of viral vectors in gene therapy by testing their functional properties, such as infectivity and gene delivery efficiency.
- Avoidance of immune response. Ensure proper handling of viral vectors to minimize host immune response to the vectors, thereby improving safety and efficacy in subsequent applications.
- Regulatory requirements. Viral vector testing of gene therapy formulations fulfills the requirements of regulatory agencies for quality control of gene therapy products to ensure that the product can pass the approval process.
Explore Our Gene Therapy Formulation Viral Vector Testing
Purity and Integrity Testing of Viral Vectors
The purity and integrity of viral vectors are important indicators for assessing their quality. We often use SDS-PAGE analysis, Western Blotting and other analytical methods to assess the purity and integrity of viral vectors.
Determination of viral vector titer
Viral titer refers to the concentration of viral vectors, including the total number of infectious viral particles and empty capsids. Our commonly used assays include quantitative reverse transcription PCR (RT-qPCR), enzyme-linked immunosorbent assay (ELISA), and flow cytometry (FC).
Bioactivity assay of viral vectors
The biological activity of viral vectors refers to their ability to infect target cells and deliver genetic material. We generally assess the infectious ability of viral vectors through infectivity assays. In addition to using reporter gene expression analysis, the activity of viral vectors is assessed by detecting the expression of reporter genes.
Replicative virus detection of viral vectors
Replicating viruses are viruses with self-replicating ability that may be produced during the production process, which poses a threat to safety. We often utilize the indicated cell culture method and PCR or q-PCR method to assess the presence of replicating viruses.
Genomic sequence integrity testing of viral vectors
The efficacy of gene therapy depends on maintaining the integrity of the therapeutic gene sequences delivered by viral vectors. Viral vector genomic sequence integrity is guaranteed by our cutting-edge sequencing method.
Our Process of Gene Therapy Formulation Viral Vector Testing
Sample preparation
Collect viral vector samples for proper dilution and pretreatment.
Physical characterization
Particle size and number analysis using nanoparticle tracking analysis (NTA) and other techniques.
Functional analysis
Evaluate the infection ability and gene expression of viral vectors using RT-qPCR and other methods.
Purity and integrity testing
Detect the purity and integrity of viral vectors by SDS-PAGE, Western blot and other methods.
RCL/RCR assay
Detect the presence of replicating viruses using methods such as indicated cell culture, ELISA, PCR/q-PCR or PERT.
Sequence validation
Confirmation of the integrity and accuracy of the viral vector genome sequence by Sanger sequencing or next generation sequencing (NGS) technology.
Quality control
Test results are analyzed to ensure that the quality of the viral vectors meets standards.
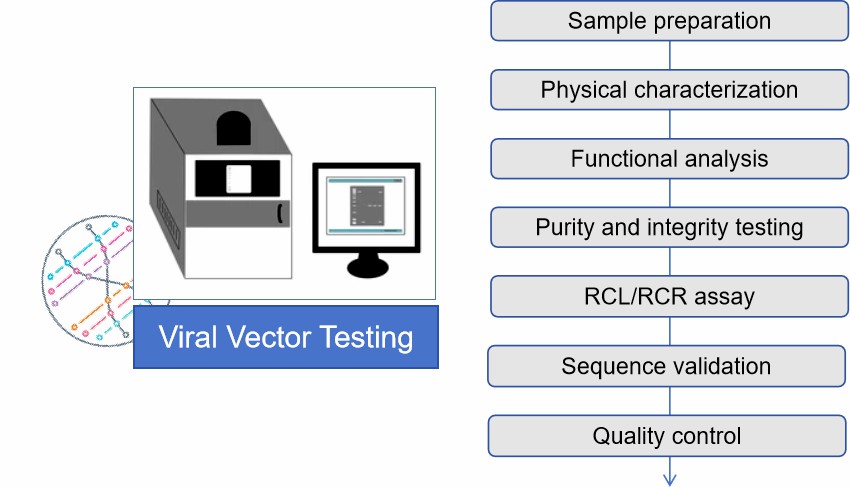 Fig.2 Viral vector testing process. (CD Formulation)
Fig.2 Viral vector testing process. (CD Formulation)
Our Technologies for Viral Vector Testing
| Platforms & Technologies |
Content Description |
| Flow cytometry (FACS) |
Flow cytometry is a technique for rapid, quantitative and multiparametric analysis at the individual cell level. By detecting the expression of reporter genes, such as green fluorescent protein (GFP), the infection activity of viral vectors can be assessed. |
| Nanoparticle tracking analysis (NTA) |
Nanoparticle tracking analysis (NTA) is a non-contact measurement method based on optical principles, in which a laser beam irradiates nanoparticles, causing the particles to scatter light, the scattered light is converted into an electrical signal by a photoelectric converter, and then information such as the size, concentration, and motion state of the particles is analyzed by a signal processing system. |
| Virus counter (VC) |
The virus counter (VC) is a flow cytometer-like device for rapid quantification of viral vectors.The VC quantifies the concentration of viral vectors by measuring their light scattering properties. |
| Quantitative reverse transcription PCR (RT-qPCR) |
RT-qPCR is capable of accurately determining the DNA copy number of viral vectors and assessing viral titer and genomic sequence integrity. |
Highlights of Our Viral Vector Testing
- Comprehensive testing services. We provide comprehensive testing services for quality control of gene therapy formulations, which greatly satisfy the various research needs of our clients.
- Technical expertise. We can provide accurate and reliable viral vector testing services for gene therapy formulations with a professional technical team and rich industry experience.
- High standard quality control. We conduct testing in strict accordance with industry standards and regulatory requirements to ensure the safety and efficacy of viral vectors.
- Customized solutions. We can provide customized testing services according to customers' needs, including the selection of testing items and optimization of testing methods.
- Advanced technology platform. We can have advanced laboratory facilities and the latest testing equipment to support high-precision testing services.
Published Data
Technology: Viral vector testing
Journal: J Virol
IF: 5.4
Published: 2023
Adeno-associated viruses (AAVs) show great promise as gene delivery vectors for treating serious human diseases. There are over a hundred naturally occurring AAV capsid variants, which are classified into phylogenetic clades based on their sequences. While extensively researched capsids like AAV8, AAV9, and AAVrh.10 are already in clinical use and have been marketed, lesser-studied capsids may possess valuable attributes, such as varying potency, tissue targeting, and lower immunogenicity, that need further exploration. Understanding these variants can enhance the knowledge of their structure and function, open opportunities for capsid engineering, and facilitate the development of novel capsids with unique characteristics.
CD Formulation provides customers with efficient, reliable and regulatory-compliant viral vector testing services to facilitate progress in the research and development of gene therapy formulations. If you are interested in us, please feel free to contact us.
References
- Yost SA, et al. Characterization and biodistribution of under-employed gene therapy vector AAV7. J Virol. 2023, 97(11):e0116323.
Related Services


 Fig.2 Viral vector testing process. (CD Formulation)
Fig.2 Viral vector testing process. (CD Formulation)