
CD Formulation understands the challenges faced by development in the bio-pharmaceutical field and focuses on providing complete bio-pharmaceutical identification and analysis services. Based on high-resolution mass spectroscopy technology and high-efficiency chromatography separation technology, CD Formulation provides a series of complete bio-pharmaceutical analysis services, such as physical and chemical property identification of proteins, peptides, antibodies, vaccines and other biological products, biological activity analysis, purity analysis, protein content, residual impurity analysis, microbial analysis, stability, and analytical method development and methodology validation, aiming to provide high-quality bio-pharmaceutical analysis services and help bio-pharmaceutical manufacturers improve the quality of bio-pharmaceuticals.
Why to Perform Analytical Testing for Bio-pharmaceuticals?
Bio-pharmaceuticals are the research and development of biomedical technology products through modern medical research methods combined with biological production technology, mainly including proteins, peptides, antibodies, vaccines, and biological diagnostic reagents and other products. However, due to the complex biological processes and numerous influencing factors of biological products, new impurities may be introduced during the production process of biological products, or the structural properties of biological products themselves may become non-uniform. These factors may weaken the efficacy of biological drugs and even cause adverse consequences such as biological toxicity and immune response. Therefore, before biological drugs enter clinical testing, they must undergo a series of rigorous tests to determine whether the biological drugs are compliant.
Our Analytical Testing Services
CD Formulation, as a leading bio-pharmaceutical laboratory services company, provides you with the comprehensive, innovative and timely solutions and helps you quickly complete your bio-pharmaceuticals analysis requirements. We support all phases of the bio-pharmaceuticals development process through our extensive technical expertise in biochemistry, molecular and cell biology, chemistry and microbiology.
Specific information on our services is provided in the following table.
| Items |
Specific Analysis Services |
| Structural Characterization and Confirmation |
- Amino Acid Sequence Analysis
|
- Amino Acid Composition Analysis
|
- Terminal Amino Acid Sequence Analysis
|
|
- Sulfhydryl Group(s) and Disulfide Bridges Analysis
|
- Carbohydrate Structure Analysis
|
| Physicochemical Properties |
- Molecular Weight or Size Determination
|
- Isoform Pattern Determination
|
- Extinction Coefficient (or Molar Absorptivity) Determination
|
|
- Liquid Chromatographic Patterns
|
|
| General Testing Services |
|
|
|
|
- Subvisible Particulate Matter
|
|
|
|
|
| Protein Content Determination |
- Protein Content Determination (Poly-peptide, Nucleic Acid)
|
| Purity Analysis Services |
|
- Nucleic Acid Purity Analysis
|
| Impurity Determination |
- Process-Related Impurity Analysis
|
- Product-Related Impurity Analysis
|
| Contaminants Analysis |
|
|
|
- Bacterial Endotoxin Assay
|
|
| Biological Activity (Potency) Testing Services |
|
| Stability Testing |
- Influencing Factor Testing
|
- Accelerated and Long-term Testing
|
| Analytical Method Validation of Bioactivity and Potency |
- Analytical Method Validation of Bioactivity and Potency
|
| Additional testing of special dosage forms |
- Other additional testing of special dosage forms
|
CD Formulation, as your service provider of choice, has broad-based and professional knowledgeable resources and a fully equipped laboratory that retains intellectual property for our clients and the flexibility to meet your specific requirements. In addition to the above-mentioned special services, we can also provide you with other additional testing of bio-pharmaceuticals analysis that you require. If you are interested in our services, please do not hesitate to contact us for in-depth discussions.
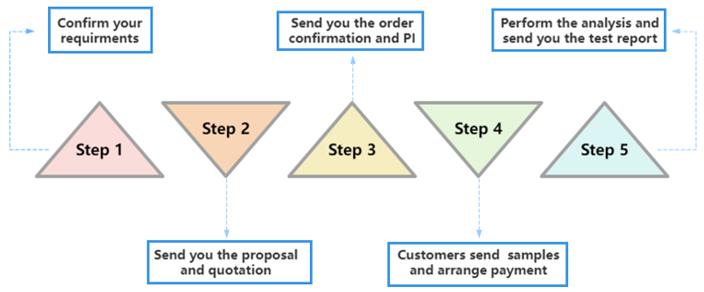
Our Workflow for Analytical Testing Services
Advantages of Our Services
- We offer a comprehensive range of professional, unbiased, high-quality measurement services with a strong commitment to providing bio-pharmaceuticals analytical services that are meaningful, impactful and at the forefront of science.
- With advanced testing instruments and equipment, with a strong and elite team of experts.
- Able to provide quick response to your bio-pharmaceutical analysis requirements immediately.
- All experiments are signed confidentiality agreements, focusing on protecting customer privacy.
How to Contact Us?
If you have a requirement about our services, please contact us by phone or email, our colleagues will reply to you within three working days.
Related Services




