Structural Characterization and Confirmation
InquiryCD Formulation has more than ten years of experience in protein spectrometry and characterization of biological macromolecules. It has a world-class protein spectrometry analysis platform. Develop and establish mass spectrometry characterization methods for various types of protein drugs, including but not limited to monoclonal antibodies, fusion proteins, bispecific antibodies, ADCs, etc.
Why to Perform Structural Characterization and Confirmation?

Therapeutic protein drugs are isolated from various biological matrices and are generally heterogeneous mixtures of closely related isomers of different molecular weights and different charges. They are derived from living cells and often contain a variety of product- and process-related impurities. In addition, recombinant proteins often undergo complex post-translational modifications, have highly specific three-dimensional structures that are partially dependent on disulfide bonds, and may aggregation, adsorption, and truncation occur. Therefore, comprehensive chemical, physical, and conformational characterization are necessary to understand the heterogeneity, impurity profile, and effectiveness of recombinant protein pharmaceuticals. Accurate amino acid sequence, molecular weight, valency changes, glycosylation, aggregation levels, and oxidation levels are all key elements in protein drug characterization.Therefore, accurate and reproducible characterization methods are indispensable to support and guide drug production and formulation decisions.
Our Services for Structural Characterization and Confirmation
Bio-pharmaceuticals are currently the fastest-growing market segment in the pharmaceutical industry, covering therapeutic proteins, fusion proteins, monoclonal antibodies and antibody-drug conjugates (ADCs). These are all complex biological macro-molecules, with molecular weights thousands of times greater than "traditional" small molecule drugs. Bio-pharmaceuticals require highly complex analytical workflows for their analysis and characterization. CD Formulation provides comprehensive tools for bio-pharmaceutical discovery, characterization, development and control. Specific information on our services is provided as follows.
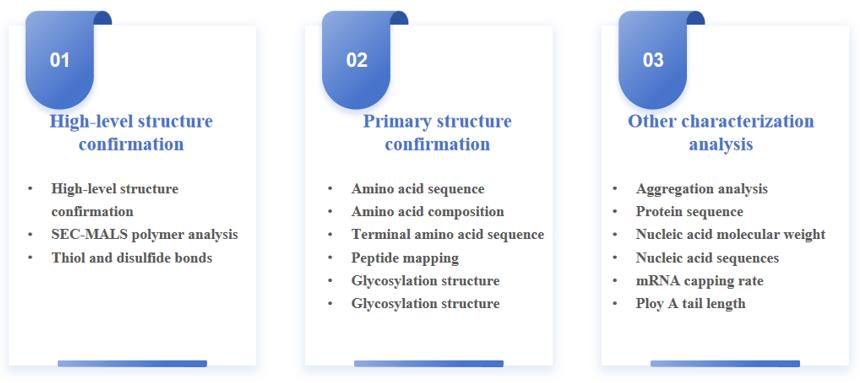
Our Workflow for Structural Characterization and Confirmation
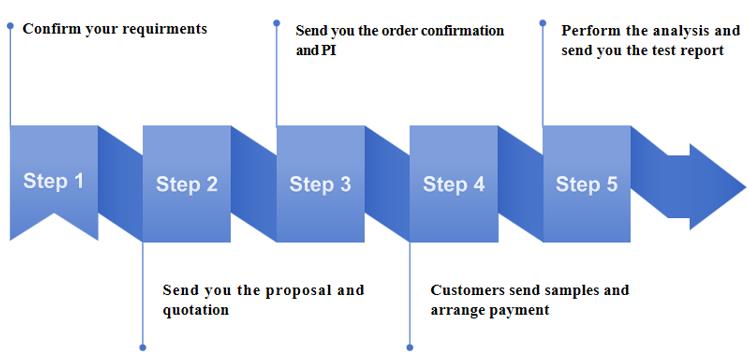
Our Advantages
- We offer a comprehensive range of professional, unbiased, high-quality measurement services with a strong commitment to providing bio-pharmaceuticals structural characterization and confirmation services that are meaningful, impactful and at the forefront of science.
- With advanced testing instruments and equipment, with a strong and elite team of experts.
- Able to provide quick response to your bio-pharmaceutical structural characterization and confirmation requirements immediately.
- All experiments are signed confidentiality agreements, focusing on protecting customer privacy.
How to Contact Us?
If you have a requirement about our services, please contact us by phone or email, our colleagues will reply to you within three working days.
Related Services





