Oral Thin Film Process Development
Inquiry
Oral thin films are most widely used because of the low cost of treatment, convenience and self-medication, due to high patient compliance. The most important aspects that should be considered include good taste for better acceptance by the patient, high moisture resistance and a suitable tension surface to withstand the stress of moisture and oral movement, good solubility in saliva, the ability to be ionized in the mouth and the ability to penetrate the oral mucosa to ensure a rapid therapeutic effect. These properties are affected by many factors in the development process of oral thin films, among which the physicochemical characteristics of APIs and excipient ingredients, and the preparation method are several important factors affecting the properties of oral thin films. CD Formulation's expert team is familiar with the development process of various oral thin films, based on our advanced technology platforms and the support of a professional R&D team, we constantly overcome difficulties, explore innovations, and provide professional technical support for process development of various oral thin films.
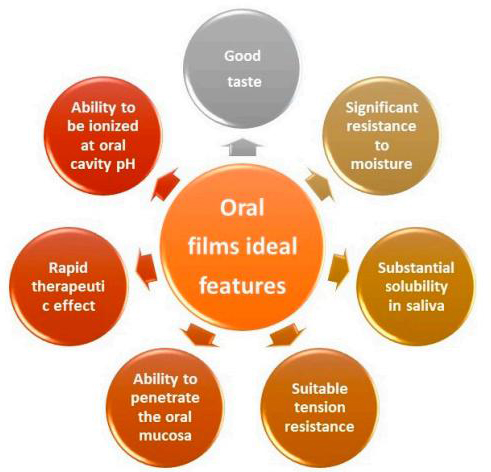 Fig.1 The ideal features of oral films. (Sevinç Özakar, R, et al., 2021)
Fig.1 The ideal features of oral films. (Sevinç Özakar, R, et al., 2021)
Advantages of Oral Thin Films
- Easy dosage with a fast onset of action.
- Easier for children, older people, and patients requiring complex care.
- Ideal for when patients are on the go.
- Can be taken without extra water.
- Possible avoidance of first-pass effect for improved bioavailability and saving costs for the active substance.
- Drug release can be customized via different types of oral thin film technologies.
Our Oral Thin Films Process Development Services
Our professional oral thin film pharmaceutical experts and laboratory instruments can bring you comprehensive technical services and solutions to help you achieve the integration of oral thin film drug development. Our oral thin film process development includes but is not limited to:
Oral thin films can carry various kinds of APIs, but due to the limitation of dosage form size, oral thin films are difficult to carry high doses of APIs. At present, we have a variety of methods to increase the drug-loading capacity of oral thin films. From the perspective of large-scale production, the uniform distribution of poorly soluble drug molecules in miscible polymers is very important, combined with our rich experience in solid-state research, the drug into a solid dispersion form, and when the water-soluble API exists in the oral membrane in a dissolved state or the form of a solid dispersion, the insoluble drug can be uniformly dispersed in the oral thin films.
The development of oral thin films requires specific functional excipient ingredients, and in specific products, the types and proportions of excipient ingredients must be adjusted to achieve the desired characteristics. We have broken through several technical barriers in the formulation process and industrialization research of oral thin films and can provide the latest generation of solid dispersion technology and taste-masking technology to further stabilize the stability of APIs and cover up the bad taste of APIs in oral thin films.
The preparation methods of oral thin films include solvent casting method, hot melt extrusion method, semi-solid casting method, solid dispersion extrusion method, etc. The most commonly used methods are the solvent casting method and the hot melt extrusion method. In addition, new technologies such as 3D printing have been better developed in the past few years. We have established advanced technology platforms for the preparation of oral thin films, and we select the most suitable oral thin films according to the physicochemical properties of customers' APIs to help customers develop high-quality orally dissolving films and accelerate the launch of drugs.
What Problems Can We Solve?
APIs Screening and Characterization
According to the type and efficacy of the oral thin films that the customer wants to develop, we help the customer screen the most suitable APIs, and perform comprehensive characterization services for the screened APIs and the customer's candidate APIs.
Excipient Ingredients Evaluation
According to the properties of APIs and the type of oral thin films, we can select the most suitable excipients, and perform a comprehensive characterization of excipients and compatibility study of APIs-excipients.
Preparation Method Screening
We can screen the preparation method based on the physicochemical properties of APIs and the types of oral thin films and evaluate the feasibility of the screened preparation methods.
Our Advantages in Oral Thin Films Process Development
- Professional Development Services: We provide compliant, high-quality, reliable, and efficient service to help partners shorten the R&D cycle, reduce trial and error costs, and accelerate product registration, and market launch.
- Advanced Oral Thin Film Development Platforms: We have a strict oral thin film drug development environment, high-tech instruments and equipment, and a complete experimental platform.
- Efficient Services: We have reliable raw materials suppliers to help you provide high-quality raw materials, shorten the customer's purchase cycle of raw materials, and accelerate the development process.
Process development is particularly important in the development of oral thin films, which directly determines the quality of the product, which requires you to have an experienced and reliable partner to help you complete this process. CD Formulation has helped many customers to complete the process development of oral thin films and has a very rich experience. If you have a requirement about our oral thin film process development services, please contact us by phone or email, and our colleagues will reply to you within three working days.
References
- Sevinç Özakar R, Özakar E. Current Overview of Oral Thin Films. Turk J Pharm Sci. 2021, 18(1):111-121.
- Jiaxiang Zhang, Anqi Lu, et al. Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies. Pharmaceutics. 2021, 13(10): 1613.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 The ideal features of oral films. (Sevinç Özakar, R, et al., 2021)
Fig.1 The ideal features of oral films. (Sevinç Özakar, R, et al., 2021)
