Excipient Ingredient Evaluation of Oral Thin Film
Inquiry
Excipient ingredients are inactive ingredients added to drugs to improve the formability, effectiveness, stability, and safety of the preparation. Oral thin films usually consist of APIs and excipient ingredients. Based on the different properties and functions of the excipient ingredients, the excipient ingredients of oral thin films are often divided into film-forming agents, plasticizers, fillers, disintegrants, etc. Additionally, for taste and appearance requirements, relevant flavors and colorants are used for optimization. Excipient ingredients play a core role in the development process of oral thin films, choosing appropriate excipient ingredients are crucial to the development of oral thin films with stable quality, accurate efficacy and high safety. CD Formulation provides a wide range of excipient ingredient evaluation services for oral thin films to help customers select the best excipient ingredients.
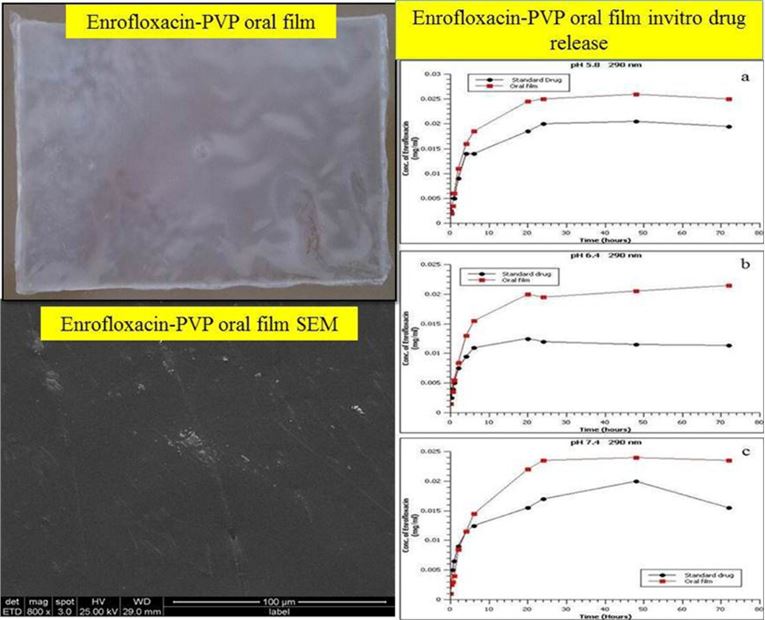 Fig.1 Film characterization and drug release. (G.Prem Kumar, A.R.Phani, et al., 2021)
Fig.1 Film characterization and drug release. (G.Prem Kumar, A.R.Phani, et al., 2021)
Types of Excipient Ingredients for Oral Thin Films
The formulation of oral thin films involves complex applications of aesthetic and performance characteristics such as taste masking, rapid dissolution, physical appearance and taste, the excipient ingredients commonly used in oral thin films are as follows:
| Film-forming agents |
Film-forming agents are the most core excipient in the oral thin films, they are used in the largest proportion, with a regular proportion of 30% to 50%. Commonly used polymers include HPMC, CMC, PVP, pectin, sodium alginate, HPC, etc. We will choose the appropriate film-forming agents according to the development requirements of customers for oral thin films. |
| Plasticizers |
Plasticizers are an important component of the oral thin film formulations. which help improve the flexibility and reduce the brittleness of oral thin films and significantly improve the performance of oral thin films by lowering the glass transition temperature of polymers. The choice of plasticizer will depend on its compatibility with the film-forming materials and the type of solvent used to prepare the oral thin films. |
| Disintegrants |
FDA recommends that the disintegration time of oral thin films in the oral cavity should be less than 30 seconds. Therefore, the use of disintegrants is to accelerate the disintegration of oral thin films in the oral cavity. |
| Colorants |
The main function of the colorants is to optimize the appearance of the oral thin films to improve the patient's compliance. The appropriate addition of colorants and aromatic agents have great significance in the use of children's medicine. |
| Fillers |
Fillers are used less frequently in oral thin films. When the size of the film is too small, which is not conducive to film forming or clinical administration, a certain amount of filler will be added to increase the film weight, or the film forming material is used alone, a certain amount of filler is needed to improve the film forming effect. |
| Saliva stimulants |
The purpose of using saliva stimulants is to increase the rate of saliva production, which will help the mouth membrane break down faster |
| Flavours |
Flavours are used to improve the taste of the film and improve patient compliance, especially for children |
| Mast-making |
Due to the special administration method of oral thin films, they remain in the oral cavity for a long time, and the taste directly affects the user's experience. Therefore, it is important to study the taste of oral thin films. Sweeteners and aromatics are usually used together to mask the bad taste of the oral thin films, both of which are food additives, and the amount is very small. |
Our Excipient Ingredient Characterization Services
As an important component of oral thin films, the quality of excipient ingredients directly affects the quality of the final drug. CD Formulation provides a wide range of excipient ingredient characterization services to support the development of your oral thin films.
Physicochemical Characterization
We analyze the particle size, surface area, shape, density, melting point, solubility and chemical composition of the excipient ingredients to be used in the oral thin films to ensure that their physical and chemical properties meet the quality requirements.
Mechanical Properties Test
We test the mechanical properties of excipient ingredients such as disintegrators and fillers to ensure that they have proper mechanical properties.
Hygroscopicity and Stability Test
We test the hygroscopicity of excipient ingredients to ensure stability during storage and use.
Toxicity and Safety Assessment
We evaluate the potential toxicity, allergy and safety of excipient ingredients through in vivo and in vitro studies.
Compatibility Study of API and Excipient Ingredients
We test the compatibility of APIs and excipient ingredients in the oral thin films to ensure the stability and effectiveness of the final product.
Our Advantages of Excipient Ingredients Evaluation for Oral Thin Films
- We will evaluate excipient ingredients from the aspects of safety, effectiveness, stability, economy and compliance to help customers select the most suitable excipient ingredients.
- In the preparation process of oral thin films, we will focus more on systematically studying the characteristics of excipient ingredients, careful thinking and observation.
- We always combine the critical quality attributes (CQAs) of excipient ingredients with the formulation composition and production process of the finished product, and comprehensively evaluating excipient ingredients that meet quality requirements.
Published Data
Technology: Essential components of orally disintegrating films and theircritical material attributes.
Journal: Current Applied Polymer Science
IF: 3.1
Published: 2019
Results: This review summarizes the research works done to address gaps in varied therapeutic areas with an emphasis on critical material attributes of its polymeric components and the critical process parameters to be considered for manufacturing robust good quality medicinal films.
CD Formulation is committed to continuously improving our technology and services to ensure that we are always at the forefront of the oral thin film excipient ingredients evaluation industry. If you have any requirements regarding our evaluation of excipient ingredients for oral thin film services, please contact us by phone or email. Our colleagues will respond to you within three working days.
References
- Suhani Sinha, Rohit Dutt. Oral Soluble Films: Attributes of the Polymeric Material and Critical Process Parameters for Designing Good Quality Films. Current Applied Polymer Science. 2019:167-188.
- G. Prem Kumar, A.R. Phani, et al. Polyvinylpyrrolidone oral films of enrofloxacin: Film characterization and drug release. International Journal of Pharmaceutics. 2024,Vol(471):146-152.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Film characterization and drug release. (G.Prem Kumar, A.R.Phani, et al., 2021)
Fig.1 Film characterization and drug release. (G.Prem Kumar, A.R.Phani, et al., 2021)
