APIs Screening and Characterization of Oral Thin Films
Inquiry
Active pharmaceutical ingredient (API) is the main component of oral thin films to exert therapeutic effect. During the pre-formulation phase of oral thin films, determining the physicochemical properties of APIs through reliable characterization analysis is essential to ensure that pharmaceutical properties are always maintained. This helps determine drug formulation options and develop superior drug delivery systems to support drug stability and efficacy. The expert team at CD Formulation has extensive experience in screening and characterizing APIs for oral film. We perform a detailed evaluation based on the type of oral film the customer is developing and the properties of the candidate APIs. This ensures that the customer will create an oral thin film with precise efficacy, significant therapeutic effects, and minimal side effects.
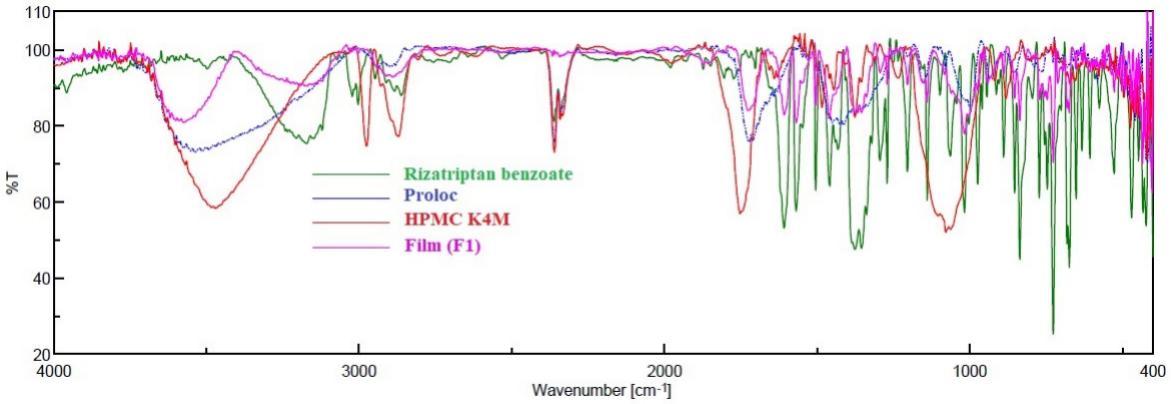 Fig.1 FTIR spectra of rizatriptan, Proloc, HPMC F4M and drug-loaded buccal film (F1). (Anroop B. Nair, et al., 2021)
Fig.1 FTIR spectra of rizatriptan, Proloc, HPMC F4M and drug-loaded buccal film (F1). (Anroop B. Nair, et al., 2021)
Our API Screening Services of Oral Thin Film
Due to the small size of oral thin films and their limited drug loading capacity, formulation development for these films can be quite challenging. Additionally, if the raw material drug has poor water solubility, it will need to be solubilized. We will tailor our strategies to the specific characteristics of different APIs and embed them in the thin film matrix to enhance drug load and improve solubility. We will also assist in selecting the most suitable APIs based on the type and efficacy of the oral thin films desired by customers, in order to accelerate the development and launch of their products.
Our API Characterization Services of Oral Thin Film
API characterization is the process of evaluating and defining the physicochemical and biological properties of active pharmaceutical ingredients. This may include chemical structure identification, purity, solubility, fluidity, particle size, stability, hygroscopicity, viscosity, and bioactivity testing of the APIs. CD Formulation is a leading provider of API characterization, offering a wide range of API characterization and testing services to support your oral thin film development.
Our Capabilities for API Characterization
| Appearance |
By visually inspecting the API's color, clarity, and physical form (e.g., powder, crystal, or solid). |
| Solubility |
The solubility of the APIs is determined by measuring the dissolution of the API in different solvents and temperatures. |
| Melting point/range |
The properties and purity of the APIs are obtained by measuring the temperature at which it changes from a solid to a liquid state. |
| Particle size distribution |
The particle size distribution of APIs affects the dissolution rate, bioavailability and stability of the final drug product. Techniques such as sieving analysis, laser diffraction or microscopy are used to perform particle size distribution tests. |
| Density |
The density of the APIs can affect the dosage form design and manufacturing process, and the density analysis of the API is also critical. |
| Hygroscopicity |
Evaluating the hygroscopicity of the APIs help determine the appropriate handling conditions and ensure the quality of the final drug. |
| Water content |
Excessive moisture can affect the stability and quality of the APIs, so it is also important to evaluate the moisture content, usually using techniques such as Karl Fischer titration or weightless drying. |
| Flow characteristic |
Assessing the flow characteristics of the APIs are essential for proper handling during manufacturing. Techniques such as Angle of rest, flow rate or compression index can be used to assess flow characteristics. |
| pH |
The pH of an API is important for assessing its chemical stability and compatibility with other ingredients or dosage forms. |
| Chemical structure |
Nuclear magnetic resonance, Fourier transform infrared and near-infrared absorption spectroscopy are usually used to determine the chemical structure, quantify the residual solvent of APIs, and identify the functional groups in the molecular structure. |
Why Choose Us to Screen and Characterize the API of Oral Thin Film?
- Our long-standing expertise in the screening and characterization of APIs helps you find, screen, test and customize APIs to meet your needs.
- We can help you to optimize an experiment based on the most cost-effective strategies and provide you the deep data analysis based on descriptive and inferential statistics. We will help you to know your materials better and provide you the solutions to solve your problems from every aspect.
- Quick turnaround time.
- The design of experiments follows the principles of statistics. The quality and accuracy of analysis results are taken seriously.
Published Data
Technology: Preparation, Characterization, and Evaluation of Cytotoxicity of Fast Dissolving Hydrogel Based Oral Thin Films
Journal: Gels
IF: 4.6
Published: 2023
Results: In this study, a quantification method for PG and MC was developed and validated simultaneously. Optimum formulations were selected with organoleptic and morphological controls, moisture absorption capacity, swelling capacity, percent elongation, foldability, pH, weight variability, thickness, disintegration time, and transparency tests on OTFs prepared by the solvent pouring method. Content uniformity, dissolution rate, determination of release kinetics, SEM, XRD, FT-IR, DSC, long-term stability, and cytotoxicity studies on the tongue epithelial cell line (SCC-9) were performed on selected OTFs.
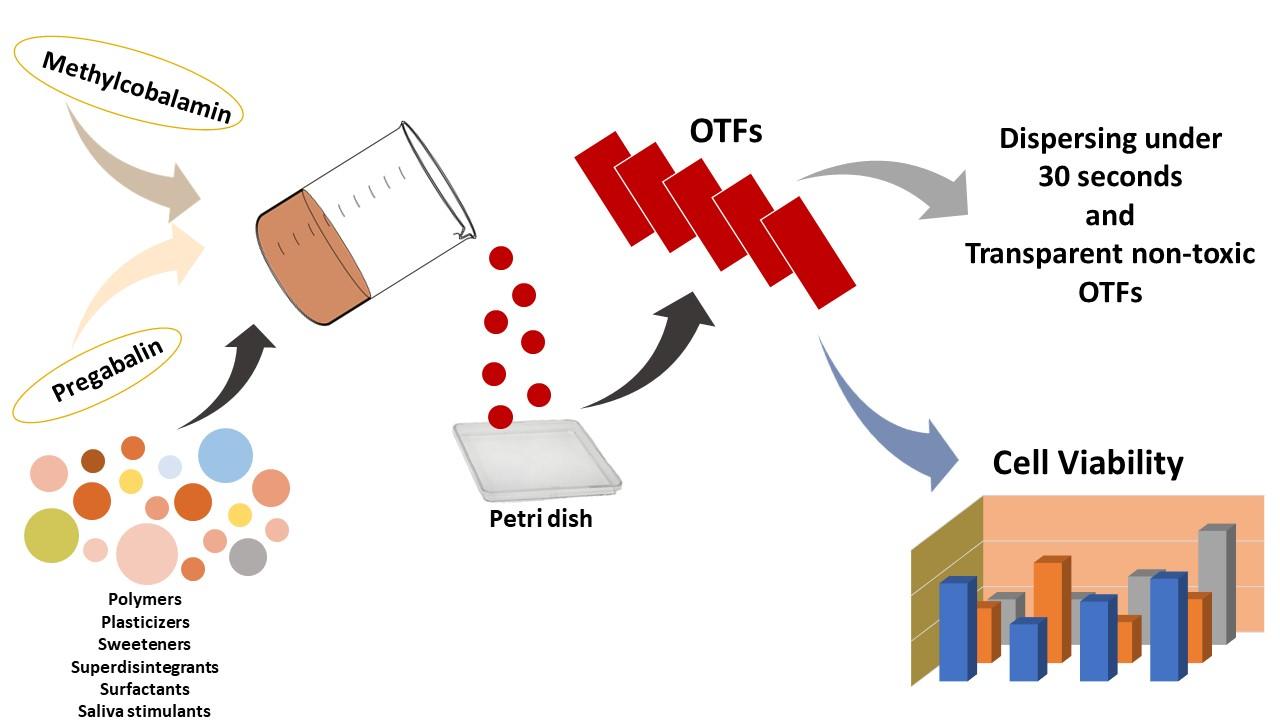 Fig.2 Preparation, Characterization, and Evaluation of Cytotoxicity of Fast Dissolving Hydrogel Based Oral Thin Films. (Emrah Özakar, et al., 2023)
Fig.2 Preparation, Characterization, and Evaluation of Cytotoxicity of Fast Dissolving Hydrogel Based Oral Thin Films. (Emrah Özakar, et al., 2023)
Screening and characterization of APIs are an important part of the quality control process, which are important for product formulation, quality control purposes, verifying raw material specifications and helping to resolve issues related to failures. CD Formulation provides a wide range of APIs screening and characterization services to support your oral film development. If you have any requirements for our APIs screening and characterization of oral thin films services, please contact us by phone or email and one of our colleagues will get back to you within three working days.
References
- Muhammad Irfan, Sumeira Rabel, et al. Orally disintegrating films: A modern expansion in drug delivery system. Pharmaceutics. 2016, Vol (24):537-546.
- Anroop B. Nair, Jigar Shah, et al. Development of Mucoadhesive Buccal Film for Rizatriptan: In Vitro and In Vivo Evaluation. Pharmaceutics. 2021:2-16.
- Emrah Özakar, Rukiye Sevinç-Özakar, et al. Preparation, Characterization, and Evaluation of Cytotoxicity of Fast Dissolving Hydrogel Based Oral Thin Films Containing Pregabalin and Methylcobalamin. 2023, 9(2).
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 FTIR spectra of rizatriptan, Proloc, HPMC F4M and drug-loaded buccal film (F1). (Anroop B. Nair, et al., 2021)
Fig.1 FTIR spectra of rizatriptan, Proloc, HPMC F4M and drug-loaded buccal film (F1). (Anroop B. Nair, et al., 2021) Fig.2 Preparation, Characterization, and Evaluation of Cytotoxicity of Fast Dissolving Hydrogel Based Oral Thin Films. (Emrah Özakar, et al., 2023)
Fig.2 Preparation, Characterization, and Evaluation of Cytotoxicity of Fast Dissolving Hydrogel Based Oral Thin Films. (Emrah Özakar, et al., 2023)
