Lyophilized Liposome Reconstitution Analysis
Inquiry
Lyophilization is a common technique used to improve the physical and chemical stability of parenteral liposome products. Liposome solutions or suspensions undergo freezing, primary drying (sublimation), and secondary drying processes to remove water and/or solvents, leaving the pharmaceutical substance and excipients in dry form as freeze-dried cakes. Cake appearance, content, impurities, reconstruction time, and residual water/solvent are typical key quality attributes (CQA) of lyophilized pharmaceutical products. CD Formulation is equipped with an advanced technology platform for the reconstruction research of lyophilized liposomes, and we are committed to providing professional solutions for our clients.
Why Conduct Lyophilized Liposome Reconstitution Analysis?
Lyophilization is one of the most effective ways to preserve liposomal drugs for a long time and minimize contamination and decomposition. As a result, many biopharmaceuticals that require rigorous storage are sold globally in powder form. However, freeze-drying and restoration may bring significant drug changes to biopharmaceuticals, such as changes in physicochemical properties, protein denaturation or degradation, and aggregation, leading to loss of therapeutic efficacy and safety concerns. In addition, it is important to select a suitable complex solution, such as sterile water for injection, 0.9% sodium chloride injection, or 5% glucose injection, as lyophilized liposomal drugs may be unstable for some complex solutions in some cases. Therefore, it is crucial to investigate the reconstituted structure and drug activity of freeze-dried liposome-based drugs for their development.
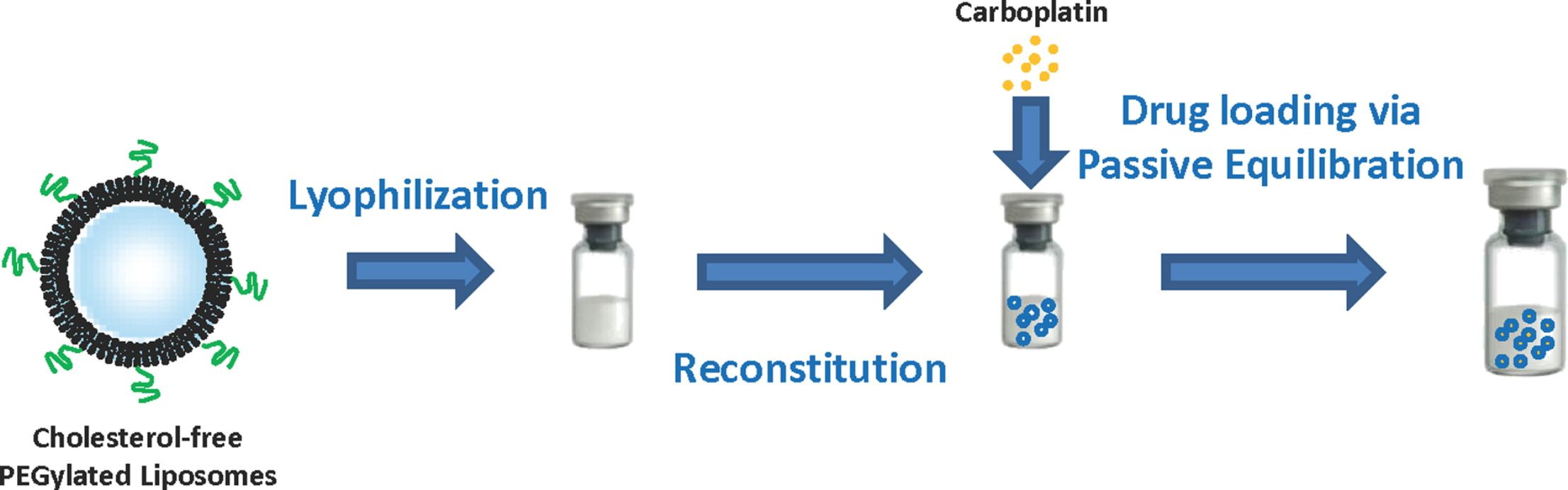 Fig.1 Liposome reconstruction diagram. (Anumita Chaudhury, et al., 2012)
Fig.1 Liposome reconstruction diagram. (Anumita Chaudhury, et al., 2012)
Our Services for Lyophilized Liposome Reconstitution Analysis
Lyophilized liposome reconstitution is the last step before use. Therefore, it is very important to characterize the changes in its stability and physicochemical properties. Therefore, we provide you with the following types of services:
Reconstructed Solution Screening
We offer sterile water for injection, 0.9% sodium chloride injection, and 5% glucose injection as complex solutions because they are clinically common and widely used.
Structure Analysis after Reconstitution
We offer to scan a variety of analytical techniques to evaluate the structure before and after lyophilization and to evaluate the microstructure transformation after reconstruction, Such as electron microscopy (SEM), Brunauer, Emmett & Teller (BET) specific surface area measurement, mercury intrusion porosimetry (MIP), X-ray micro-computed tomography (micro-CT) technique.
Physical and Chemical Characteristics Analysis
During the reconstruction, the stability of lyophilized liposomes after reconstruction will be characterized by the physicochemical transformation of the reconstructed solution, such as particle size distribution, potential transformation, encapsulation rate transformation, drug leakage rate, drug impurity transformation, etc.
Reconstruction Process Research
Accurate determination of recovery time is essential to aid formulation development and support product quality control. Traditional methods of quantifying recovery time involve visual identification of endpoints, which leads to varying values reported from studies. The endpoint is determined by visual observation of the clarified solution conforming to the pharmacopeia guidelines. Instead of traditional methods, we use serial spectral analysis techniques to study the reconstruction time endpoint. In addition, we provide stirring frequency research services during reconstruction.
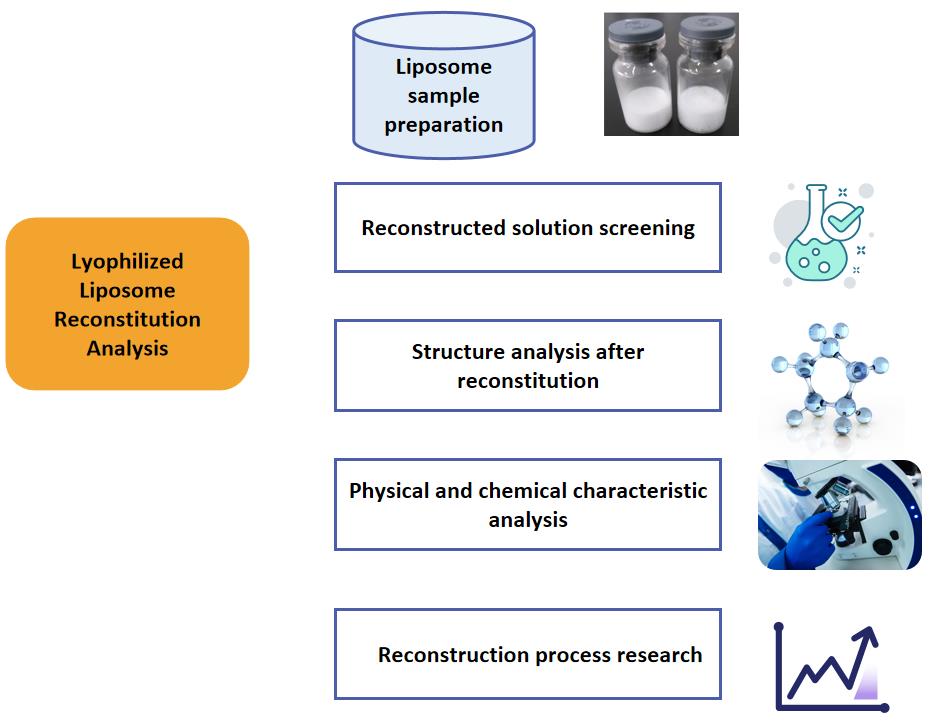 Fig.2 Workflow of lyophilized liposome reconstitution analysis. (CD Formulation)
Fig.2 Workflow of lyophilized liposome reconstitution analysis. (CD Formulation)
Our Platforms for Lyophilized Liposome Reconstitution Analysis
| Techniques |
Detailed Information |
| Brunauer, Emmett & Teller (BET) specific surface area technique |
- BET technically refers to the theory used to determine the specific surface area but is often extended to refer more broadly to the surface area and aperture measured by gas adsorption.
- The analysis is based on the physical adsorption of inert gases, such as nitrogen and krypton, on the solid surface of the sample. The measured specific surface area is measured in m2/g.
|
| Mercury intrusion porosimetry (MIP) technique |
- A technique used to measure pore size and volume in porous solid and powder materials. It uses non-wetting liquid mercury to force its way into solid pores under pressure. The pressure required to push mercury into the pores of the sample is inversely proportional to the size of the pores.
|
| X-ray micro-computed tomography (micro-CT) technique |
- A non-destructive imaging technique that uses X-rays to create 3D images of small objects or samples.
- It is similar to a medical CT scanner but is designed for smaller specimens, usually a few millimeters to a few centimeters.
|
| Reconstruction process research platform |
- The changes in resolution time, resolution process, and physicochemical properties are systematically studied to help the development and characterization of lyophilized liposomes.
|
Our Key Advantages in Lyophilized Liposome Reconstitution Analysis
- Advanced facilities. We offer professional facilities and technology platforms for Lyophilized liposome reconstitution analysis commitment to customer satisfaction, including BET technique, micro-CT technique, MIP technique, etc.
- Scientific and cost-effective. Strictly implement industry analysis standards and design scientific experimental programs. A tailor-made experimental program for customers to solve every question of customers.
- Professional team. We have a professional technical team dedicated to liposome reconstitution services by freeze-drying. Our team of scientists has professional technical expertise and extensive experience in characterizing liposomes pre- and post-freeze-drying, as well as in investigating reconstitution processes.
Published Data
Technology: Lyophilized liposome reconstitution analysis technique
Journal: International Journal of Pharmaceutics.
IF: 10.5
Published: 2023
Results: The aim of this study was to develop an optimized lyophilized liposome formulation containing a lyophilized protective agent, considering factors such as the type and concentration of the lyophilized protective agent to ensure that the finished product has the appropriate pH-sensitive lyophilized liposome formulation to exert anticancer activity against HER2-positive cancers. The effects of carbohydrates on the stability of liposomes were studied based on the particle size analysis of liposomes. The morphology of liposomes was studied by transmission electron microscopy (TEM) and cryogenic transmission electron microscopy (cryo-TEM). Freeze-dried liposome formulations of 5-Dox (containing trehalose) showed excellent stability and release properties when stored at 4°C and reconstructed in PBS after 120 days.
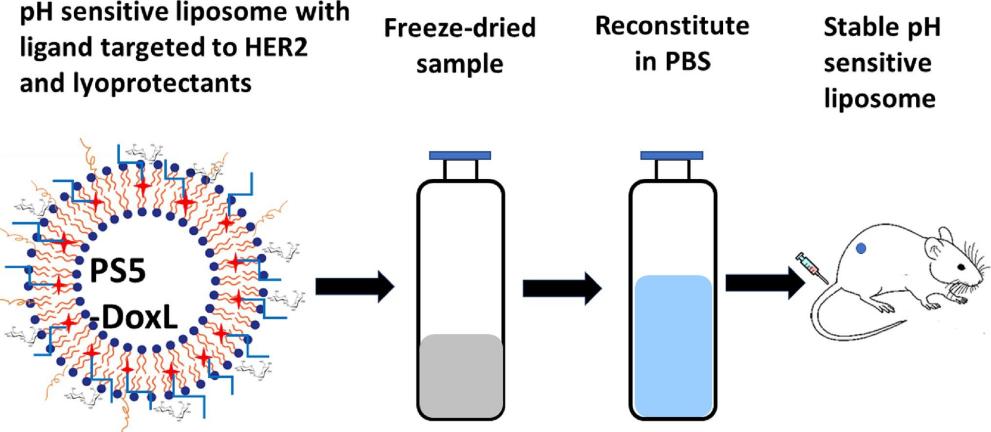 Fig.3 Lyophilized liposome reconstitution study workflow. (Jafrin Jobayer Sonju, et al., 2023)
Fig.3 Lyophilized liposome reconstitution study workflow. (Jafrin Jobayer Sonju, et al., 2023)
CD Formulation has an advanced lyophilized liposome analysis platform, aiming to provide customers with professional solutions. If you need any help, please contact us immediately.
References
- Anumita Chaudhury, Surajit Das, et al. Lyophilization of cholesterol-free PEGylated liposomes and its impact on drug loading by passive equilibration. International Journal of Pharmaceutics, 2012, Volume 430, Issues 1–2, Pages 167-175.
- Jafrin Jobayer Sonju, Prajesh Shrestha, et al. Lyophilized liposomal formulation of a peptidomimetic-Dox conjugate for HER2 positive breast and lung cancer. International Journal of Pharmaceutics. 2023. Volume 639.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Liposome reconstruction diagram. (Anumita Chaudhury, et al., 2012)
Fig.1 Liposome reconstruction diagram. (Anumita Chaudhury, et al., 2012) Fig.2 Workflow of lyophilized liposome reconstitution analysis. (CD Formulation)
Fig.2 Workflow of lyophilized liposome reconstitution analysis. (CD Formulation) Fig.3 Lyophilized liposome reconstitution study workflow. (Jafrin Jobayer Sonju, et al., 2023)
Fig.3 Lyophilized liposome reconstitution study workflow. (Jafrin Jobayer Sonju, et al., 2023)
