Lyophilized Liposome Hygroscopicity Determination
Inquiry
Freeze-drying technology is widely employed to enhance the physical and chemical stability of intravenous liposome products. The appearance of lyocakes, content, impurities, reconstitution time, and residual water/solvent are typical critical quality attributes of freeze-dried pharmaceuticals. CD Formulation utilizes an advanced technology platform for determining the hygroscopicity of lyophilized liposomes and we are dedicated to delivering professional solutions for our clients.
Why Conduct Lyophilized Liposome Hygroscopicity Determination?
The main purpose of freeze-drying is to reduce the water content of the product by sublimation to achieve long-term stable preservation. However, even if the residual water content of the liposome product is controlled below the standard by freeze-drying, the problem of gradually increasing water content during the stable period is often encountered. The low water content and spongy structural characteristics of freeze-dried liposome products also contribute to their high moisture absorption capacity. Moisture absorption significantly affects product stability and shelf life, underscoring the importance of hygroscopicity analysis for lyophilized liposomes.
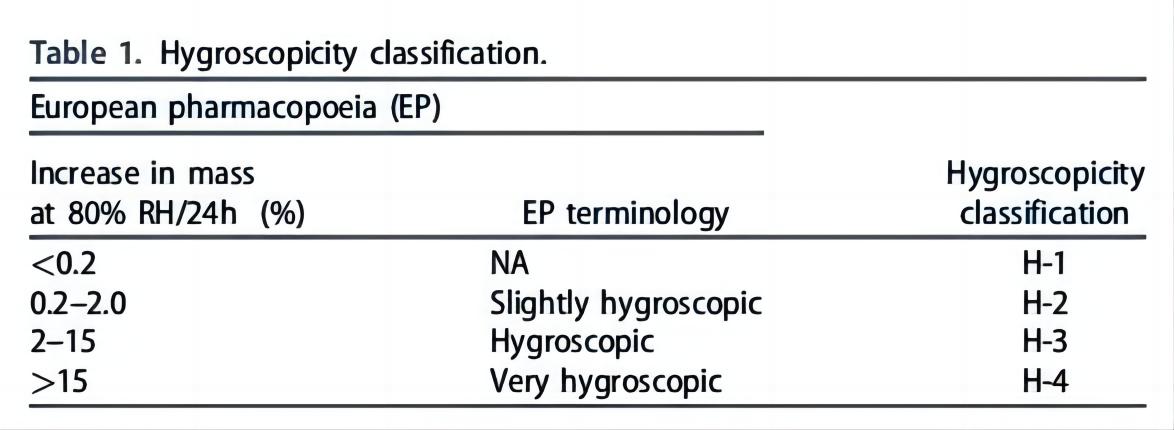 Table 1. Classification of hygroscopicity in the European Pharmacopoeia. (Chave. Mary, et al, 2022)
Table 1. Classification of hygroscopicity in the European Pharmacopoeia. (Chave. Mary, et al, 2022)
Our Services for Lyophilized Liposome Hygroscopicity Determination
The parameters used in hygroscopicity evaluation include critical relative humidity, hygroscopicity potential, hygroscopicity coefficient, and heat of adsorption. We offer the following types of services related to liposome hygroscopicity research.
Critical Relative Humidity (CRH) and Moisture Absorption Rate Study
Water-soluble drug powder is generally not hygroscopic at low relative humidity, except when the relative humidity increases to a value, the hygroscopic amount improves rapidly. The relative humidity at this time is called critical relative humidity (CRH). The hygroscopic velocity represents the hygroscopic amount per unit of time under a specific relative humidity. The determination of CRH is mainly to control the humidity of the production environment and avoid drug moisture absorption. We offer saturated solution and dry powder methods for CRH and moisture absorption rates.
Hygroscopic Curve Analysis
We provide hygroscopic equilibrium curve analysis (a graph of the relationship between hygroscopic capacity (equilibrium moisture content) and the relative humidity of the air) to provide customers with the maximum hygroscopic capacity of liposome lyophilized powder at different relative humidity.
Moisture Absorption Isotherm Analysis
We establish a moisture absorption isotherm for liposome lyophilized powders. This service connects the water adsorption isotherm to the water activity of the corresponding lyophilized material. We can provide the gravity method to obtain this curve. It is important to note that at high relative humidity, specific components/excipients in the formulation may crystallize, which can be determined by polarizing microscopy. It can also be confirmed by powder X-ray diffraction. The weight of the sample can be continuously measured at each water activity level until equilibrium is observed.
Dynamic Migration Analysis of Water
The water migration process is an important link to improving drying efficiency and drying quality. Through the sampling weighing and model prediction method, the overall moisture content of the material can be inferred, but it cannot reflect the uniformity of moisture, and the measurement results also have great errors according to the specific working conditions. We provide low-field NMR technology to study the water migration of liposome freeze-dried powder, which is also a powerful means of water molecule content, state, distribution, migration, diffusion, and interaction with other molecules.
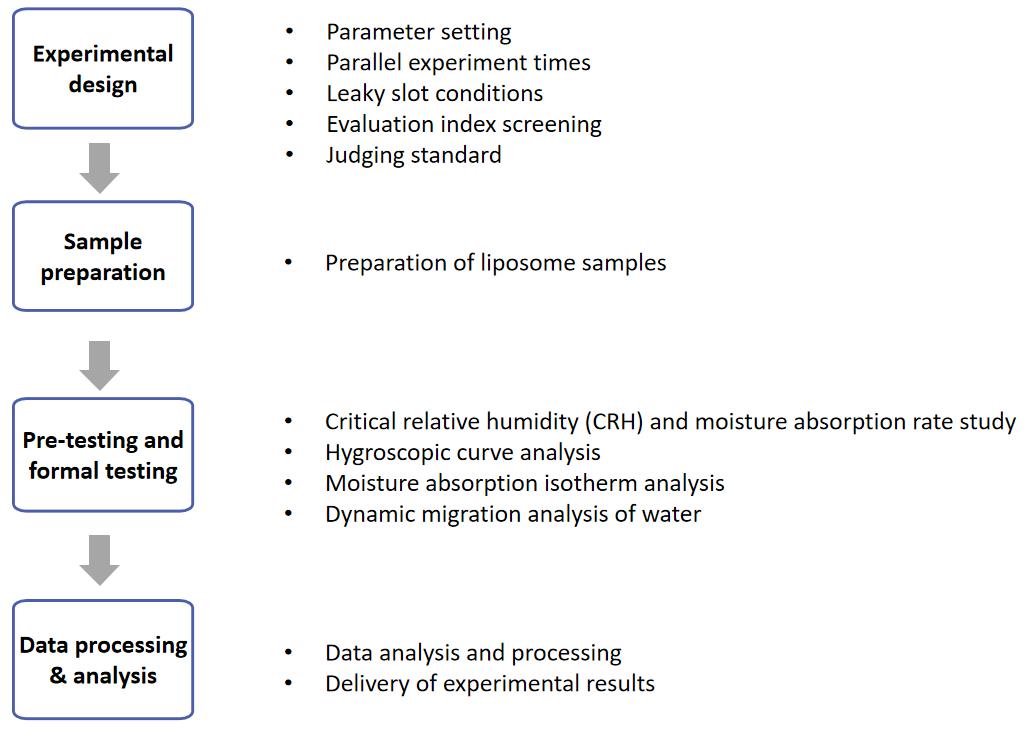 Fig.1 Workflow of lyophilized liposome hygroscopicity determination services. (CD Formulation)
Fig.1 Workflow of lyophilized liposome hygroscopicity determination services. (CD Formulation)
Our Platforms for Lyophilized Liposome Hygroscopicity Determination
| Techniques & Platforms |
Detailed Information |
| Dynamic migration analysis platform |
- Low-field nuclear magnetic resonance analysis and imaging, LF-NMR/MRI technology.
- LF-NMR/MRI technology can not only provide the total signal intensity of water, but also effectively analyze the composition and distribution of water and can reflect the drying state of lyophilized liposomes in three-dimensional space.
|
| Moisture absorption isotherm analysis & Hygroscopic equilibrium curve analysis |
- We can provide the gravity method to get a moisture absorption isotherm curve.
- To measure the solid weight change as a function of relative humidity and then convert it to a water vapor adsorption isotherm.
- Moisture absorption isotherm curve can also be applied to extract detailed information about water-solid interactions and possible polymorphic changes.
- The moisture absorption equilibrium curve has important guiding significance for predicting the storage process and conditions of lyophilized liposomes.
|
| Critical relative humidity (CRH) and moisture absorption rate study |
- We offer the following methods to obtain the CRH and moisture absorption rate:
Saturated solution method
Dry powder method |
Our Key Advantages in Lyophilized Liposome Hygroscopicity Determination
- Advanced facilities. Professional facilities and technology platforms are equipped to commit to customer satisfaction, including LF-NMR/MRI technology, gravity method, etc.
- Team of scientists. Our team of scientists is well-versed in the moisture absorption testing of freeze-dried powders, especially liposome freeze-dried powders. They have accumulated many years of analytical experience in this field, helping numerous enterprises to solve hygroscopicity problems efficiently.
- Scientific. Strictly implement industry analysis standards and design scientific experimental programs.
- Cost-effective. Our services are flexible and can be customized to meet different needs about lyophilized liposome hygroscopicity determination services.
Published Data
Technology: Lyophilized liposome water adsorption mechanism study technique
Journal: Food Research International.
IF: 12
Published: 2023
Results: In this study, the authors aimed to develop stable ibrutinib liposomes and assess their long-term stability. The mass design technique was utilized to optimize Ibu's protein speck liposome, followed by pegylation and characterization. Furthermore, the function and other parameters of cryoprotectants in the lyophilization process were evaluated to obtain a robust formulation. Stability studies for up to 6 months were conducted under various storage conditions to assess the impact of lyophilization on physicochemical properties such as hygroscopicity and solubility.
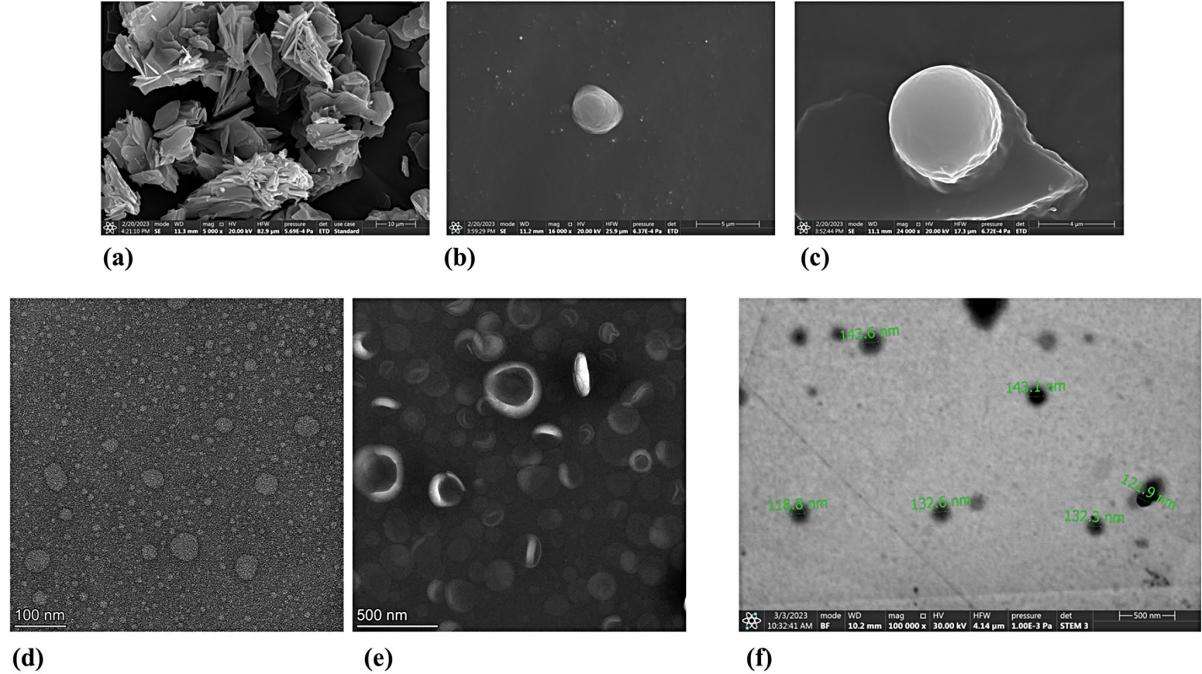 Fig.2 Morphological evaluation of lyophilized liposomes. (Leiyu Deng, et al., 2021)
Fig.2 Morphological evaluation of lyophilized liposomes. (Leiyu Deng, et al., 2021)
CD Formulation has developed a complete lyophilized liposome analysis platform and process to provide customers with tailor-made comprehensive analysis solutions, especially in lyophilized liposome hygroscopicity determination. If you need any help, please contact us immediately.
References
- Chaves. Mary, Kelly. Ron, et al. Data-driven Approach to Mitigate Quality Impact of Hygroscopic Pharmaceutical Raw Materials Throughout the Supply Chain. Pharmaceutical Development and Technology. 2022, 27. 1-51.
- Panchal, K., Reddy, A., et al. Dynamic intervention to enhance the stability of PEGylated Ibrutinib loaded lipidic nano-vesicular systems: transitioning from colloidal dispersion to lyophilized product. Drug Deliv. and Transl. Res. 2024.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Table 1. Classification of hygroscopicity in the European Pharmacopoeia. (Chave. Mary, et al, 2022)
Table 1. Classification of hygroscopicity in the European Pharmacopoeia. (Chave. Mary, et al, 2022) Fig.1 Workflow of lyophilized liposome hygroscopicity determination services. (CD Formulation)
Fig.1 Workflow of lyophilized liposome hygroscopicity determination services. (CD Formulation) Fig.2 Morphological evaluation of lyophilized liposomes. (Leiyu Deng, et al., 2021)
Fig.2 Morphological evaluation of lyophilized liposomes. (Leiyu Deng, et al., 2021)
