Adenovirus Vector Purification
Inquiry
Adenovirus is a non-enveloped linear double-stranded DNA virus with a diameter of 70-90 nm. Adenoviral vectors have a wide range of host cells and are effective in infecting both dividing and non-dividing cells. Adenoviral vectors do not integrate with the host cell genome and therefore do not carry the risk of insertional mutagenesis and have a large vector capacity. However, different serotypes of adenovirus have different capsid proteins, and the corresponding downstream purification processes need to be fine-tuned.
CD Formulation keeps abreast of the cutting edge of gene therapy research, and continuously develops and optimizes our technical methods and processes. In adenovirus vector purification process research, we can provide researchers with the best purification process according to the purpose of the experiment and the specific conditions, as well as provide the whole process of technical support services, to help researchers get the best adenovirus vector purification results.
Why Conduct Adenoviral Vector Purification?
Adenoviral vectors need to be purified when used for in vivo experiments. Because the unpurified adenoviral vector collection may contain residual viral particles, cellular debris, culture fluid and other impurity components, which will cause severe host immune response if injected into cells or animals, so we must purify the adenoviral vector when preparing it. In addition, purified and concentrated adenoviral vectors have higher purity and higher titer, which are more suitable for in vivo injection in gene therapy. In conclusion, the purification process of adenoviral vectors is studied to ensure their higher safety, efficacy and consistency in gene therapy research.
Explore Our Adenovirus Vector Purification
Adenovirus purification steps typically include cell lysis, clarification, nuclease treatment, concentration and exchange, capture chromatography, and purification chromatography. Specific purification steps can be adjusted according to specific laboratory conditions, desired viral titer, and purity requirements. We keep abreast of the latest research techniques in gene therapy continue to provide new purification methods and develop new processes to improve purification efficiency and reduce costs.
Cell lysis
Cells are lysed by physical or chemical methods to release virus particles. This may include repeated freeze-thawing, sonication, or the use of lysis buffers.
Initial clarification
Remove cell debris and unlysed cells using low-speed centrifugation. Collect the supernatant, which contains the virus particles.
Concentration and initial purification
Perform initial concentration and partial purification of the virus using ultrafiltration or precipitating agents (e.g., polyethylene glycol, PEG).
Ultracentrifugation
Further purification of the virus using ultracentrifugation to separate the virus particles based on the difference in density of the virus and impurities. This may include density gradient centrifugation using cesium chloride (CsCl).
Chromatographic purification
Application of chromatographic techniques for further purification of the virus. This may include affinity chromatography, ion exchange chromatography, hydrophobic chromatography, immobilized metal affinity chromatography (IMAC), etc. Afterward, we elute the virus using appropriate buffers depending on the chromatographic technique used and collect the eluate containing the highly purified virus.
Virus titer assay
After purification, the titer of the virus needs to be determined, which is usually done by end-point dilution to determine the number of viral particles per milliliter of viral solution.
Quality control
Multiple quality control steps are performed throughout the purification process to ensure the purity, activity, and safety of the virus.
 Fig.1 Process of adenovirus vector purification. (CD Formulation)
Fig.1 Process of adenovirus vector purification. (CD Formulation)
Our Solutions for Adenovirus Vector Purification
Ultracentrifugation
As a traditional separation method, separation is achieved by high-speed centrifugation depending on the shape, size, and isodensity point of the impurity and the viral vector. Cesium chloride (CsCl), sucrose, and iodixanol can be used as media for ultracentrifugation. The advantage of this method is that the technology is mature and can be operated in general laboratories, it is suitable for all serotypes of vectors, and the ultracentrifugation method can effectively separate empty shell particles with very similar structures.
Liquid Chromatography
Chromatographic separation is the most versatile and effective method for large-scale production of recombinant adenovirus. Currently, affinity chromatography, ion exchange chromatography, and molecular exclusion chromatography have been widely used for the separation and purification of adenovirus vectors of different serotypes.
Chemical reagent method
Often used in the concentration and precipitation of viral vectors, inorganic reagents such as sulfate, calcium phosphate, calcium chloride, or non-ionic surfactants are used to precipitate the adenovirus for initial separation.
Other methods
In the production of recombinant adenovirus, purification is the key issue. Density gradient centrifugation with CsCl is the classic method for virus purification, but to meet the needs of large-scale production, we have also explored a variety of methods, including anion-exchange chromatography, volume exclusion chromatography, hydrophobic chromatography, and immobilized zinc adsorption chromatography (IZAC).
Our Platforms for Adenovirus Vector Purification
| Platforms & Technologies |
Content Description |
| Affinity chromatography |
Affinity chromatography is a highly efficient chromatographic technique that utilizes the specialized affinity between biological molecules for separation. We can utilize the affinity of specific proteins on the surface of the virus to the stationary phase for separation in adenovirus vector purification. |
| Ion exchange chromatography |
Separation by ion exchange resin under different pH conditions based on the surface charge characteristics of virus particles. |
| Immobilized metal affinity chromatography (IMAC) technology |
If the virus has a His tag on the surface, it can be purified using nickel or other metal ion affinity chromatography. |
Highlights of Our Adenovirus Vector Purification
- We have a full-process and full-coverage quality management system in adenovirus vector development and purification.
- We have complete and advanced instruments and equipment as well as a team of experienced experts.
- We provide a one-stop service from gene synthesis, and vector construction to viral packaging process development.
- We provide comprehensive technical support services for the development of viral vectors in gene therapy.
Published Data
Technology: Adenovirus vector purification
Journal: Biochem Biophys Res Commun
IF: 3.1
Published: 2009
In this study, the investigators developed a novel purification process combining anion-exchange chromatography and immobilized metal-affinity membrane chromatography for the purification of recombinant adenovirus. Adenoviruses were first purified from clarified infectious lysates by anion-exchange chromatography using Q Sepharose XL resin, and then further purified using Sartobind IDA membrane units with Zn(2+) ions as affinity ligands. Metal affinity membrane chromatography effectively removes residual host cell impurities that co-elute with the adenovirus in the previous anion exchange chromatography step. Metal affinity membrane chromatography also separated defective adenovirus particles from the infectious adenovirus grade fraction. In addition, metal affinity membrane chromatography showed higher yields compared to conventional bead-based metal affinity chromatography. The purity and specific activity of adenoviruses prepared by this two-step chromatographic method were comparable to those prepared by conventional CsCl density centrifugation.
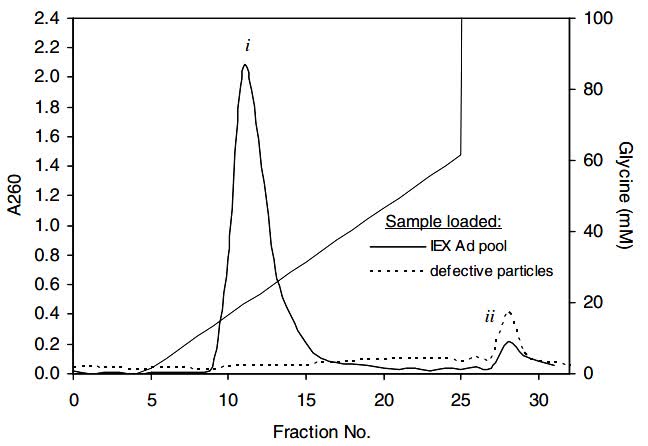 Fig.2 Metal affinity membrane chromatograms of purified adenovirus pools and CsCl purified defective adenovirus particles. (Lee DS, et al., 2009)
Fig.2 Metal affinity membrane chromatograms of purified adenovirus pools and CsCl purified defective adenovirus particles. (Lee DS, et al., 2009)
CD Formulation provides reliable technical support and solutions for viral vector purification, and this service plays an important role in the advancement of gene therapy. If you are interested in us, please feel free to contact us.
References
- Lee DS, et al. Improved purification of recombinant adenoviral vector by metal affinity membrane chromatography. Biochem Biophys Res Commun. 2009, 378(3):640-4.
Related Services

 Fig.1 Process of adenovirus vector purification. (CD Formulation)
Fig.1 Process of adenovirus vector purification. (CD Formulation) Fig.2 Metal affinity membrane chromatograms of purified adenovirus pools and CsCl purified defective adenovirus particles. (Lee DS, et al., 2009)
Fig.2 Metal affinity membrane chromatograms of purified adenovirus pools and CsCl purified defective adenovirus particles. (Lee DS, et al., 2009)