Lentivirus Vector Purification
Inquiry
Lentivirus purification is the process of isolating and purifying lentivirus. Lentiviral purification process development is crucial to ensure the safety, efficacy, and quality control of gene therapy products. It is the basis for realizing the wide application of gene therapy. CD Formulation can help researchers select the appropriate lentiviral purification solution according to the researcher's project requirements and the experiment's purpose. Over the years of viral vector purification research, we have kept pace with the development of advanced technologies and continuously improved purification and analytical methods to ensure that the fully characterized products are of high purity, efficacy, and safety.
Why Conduct Lentivirus Vector Purification?
- Improved safety. Purification removes impurities that may be present during the production of viral vectors, such as host cell proteins, DNA, and other potentially harmful substances, thereby reducing the risk to the patient when receiving treatment.
- Improved efficacy. Lentiviral vectors obtained through purification have higher purity and activity, which can improve the efficacy of gene therapy.
- Improved productivity. The development of efficient purification processes can increase the productivity of viral vectors, reduce costs and make gene therapy more economically viable.
- Optimize vector properties. The purification process may involve specific steps to optimize the properties of the viral vector, such as titer, infectivity, etc., to suit specific therapeutic needs.
Explore Our Lentivirus Vector Purification
Production preparation
Selection of suitable cell lines for expansion, which can grow in high-density conditions and can be suspension-adapted.
Plasmid transfection
Cells are transfected with lentiviral plasmids to produce lentiviral vectors. We have cationic lipid transfection reagents designed for high-density virus production in cell cultures with high transfection efficiency and low toxicity.
Virus production
Transfected cells are transferred to different culture systems such as shake flasks, shaker systems, cell factories, or bioreactors for virus production.
Clarification
After virus production, clarification is required to remove cellular debris and other large particulate matter.
Nuclease treatment
Removes plasmid DNA and host cell nucleic acid impurities.
Further purification of the virus
We use ultrafiltration and dialysis techniques to further purify the virus and improve the recovery and removal of protein and DNA.
Chromatographic purification
Chromatographic purification is performed using a multi-peak chromatography (MMC) step, which combines size exclusion and anion exchange properties in a simple and gentle process.
Sterile filtration
In the final stage of the purification process, aseptic filtration is achieved by optimizing the filter material, number of layers, etc. to maximize recovery.
Analytical testing
We ensure the quality and safety of our products through analytical testing, including sterility, identification, purity and potency testing.
 Fig.1 Process of lentivirus vector purification. (CD Formulation)
Fig.1 Process of lentivirus vector purification. (CD Formulation)
Our Solutions for Lentivirus Vector Purification
PEG concentration method
Polyethylene glycol (PEG) is an uncharged linear molecular structure of polysaccharides, a polymer of ethylene glycol, has a strong dehydrating effect, its ability to destroy the hydration layer on the surface of the protein molecule and precipitation of the protein occurs, PEG alone is used with high non-specific binding, the addition of NaCl helps to precipitate the virus.
- Basic process. Mix the lentivirus-containing supernatant and PEG well in proportion. After centrifugation, the precipitate is resuspended with virus storage solution and concentrated to a smaller volume, collected and filtered for sterilization for subsequent experiments.
- Characteristics of this purification method. Low equipment requirements and easy operation.
Ultracentrifugation
Ultracentrifugation is a method of separating, concentrating, and purifying viruses by applying strong centrifugal force with the help of an ultracentrifugal machine according to the different sedimentation coefficients, masses, and densities of substances.
- The basic process. The supernatant containing lentivirus is centrifuged to remove cellular debris, the supernatant is filtered and sterilized by membrane filtration and transferred to an ultracentrifuge tube, and after centrifugation, the precipitate is resuspended with a small amount of viral storage solution, then filtered and sterilized by membrane filtration and used for the subsequent experiments.
- Characteristics of this purification method. High purity and low toxicity of the virus.
Purification column purification method
The lentivirus purification column method is to adsorb lentivirus particles through the ionic column, and then use the elution solution to purify the virus, the resulting virus purity is high, but the cost of the experiment is also higher, and the cost-effective for routine experiments is low.
Our Platforms for Lentivirus Virus Vector Purification
| Platforms & Technologies |
Content Description |
| Stable transfection cell production platform |
This technology platform provides a plasmid-free approach to lentiviral vector production, saving on plasmid costs and transfection reagent costs, reducing operational complexity and significantly improving process robustness. |
| Size exclusion chromatography (SEC) purification process |
SEC is a technique used to purify lentiviral vectors by separating viral particles through physical size differences. In one study, SEC was used to purify lentiviral vectors generated from transiently transfected or stably produced cell lines, and protein composition in the purified vectors was analyzed using LC-MS/MS. |
| Sterile filtration optimization technology |
We maximize the recovery of viral vectors at the end of the purification process by optimizing the filtration material, number of layers and other factors. |
Highlights of Our Lentivirus Vector Purification
- Multiple purification methods. We provide numerous lentiviral vector purification methods according to different experimental requirements to help customers save experimental costs and choose the appropriate purification method.
- Quality control. Our lentiviral vector purification technology is subject to strict quality control, including mycoplasma, sterility, and titer testing.
- One-stop service. We provide a one-stop service platform from gene synthesis to viral packaging to meet customers' needs in gene therapy research.
- Professional team support. Our research team has decades of experience in viral vector development and research to ensure product quality and supply continuity.
Published Data
Technology: Lentivirus purification
Journal: Methods Mol Biol
Published: 2016
This study details a simple protocol for the production of lentiviral vectors, describing the triple transfection of lentiviral transfer vectors and lentiviral helper plasmids into HEK-293 cells, followed by the purification of the virosome from the cell culture medium. The current protocol is versatile and can be easily modified to meet the specific needs of researchers to produce relatively high titers of lentiviral vectors that can be used to transduce a variety of cells in vitro and in vivo.
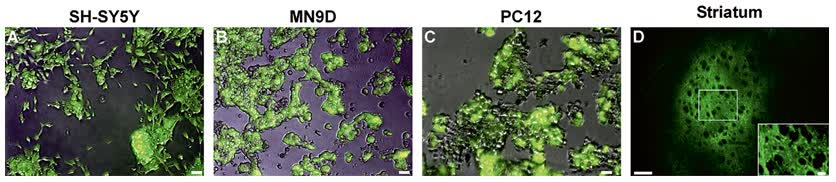 Fig.2 Expression following transduction with lentiviral vector. (Benskey MJ, et al., 2016)
Fig.2 Expression following transduction with lentiviral vector. (Benskey MJ, et al., 2016)
CD Formulation offers a full range of technical services to support researchers in the development of lentiviral vector purification processes. We sincerely hope to cooperate with you and work together to advance the research and development of gene therapy. If you are interested in us, please feel free to contact us.
References
- Benskey MJ, et al. Lentivirus Production and Purification. Methods Mol Biol. 2016;1382:107-14. doi: 10.1007/978-1-4939-3271-9_8. PMID: 26611582.
Related Services

 Fig.1 Process of lentivirus vector purification. (CD Formulation)
Fig.1 Process of lentivirus vector purification. (CD Formulation) Fig.2 Expression following transduction with lentiviral vector. (Benskey MJ, et al., 2016)
Fig.2 Expression following transduction with lentiviral vector. (Benskey MJ, et al., 2016)