Adeno Associated Virus Vector Purification
Inquiry
Recombinant adeno-associated virus (AAV) is a linear single-stranded DNA virus containing 4.7 kb and belongs to the family of small viruses. Currently, this vector is mainly for the treatment of Parkinson's disease, myotonic dystrophy, and hemophilia B, etc. Research on the improvement of the adeno-associated virus purification process plays an important role in promoting the research and application of adenoviral vectors in gene therapy. CD Formulation is an industry leader in the development of gene therapy formulations. We remain at the forefront of gene therapy research and provide researchers with optimal purification process methodologies as well as full-process technical guidance.
Why Conduct Adeno-Associated Viral Vector Purification?
Adeno-associated virus (AAV) vectors are purified to remove impurities and ensure the safety and quality of AAV-based products. Crude products of AAV vectors, such as cell lysates or culture supernatants, may contain impurities such as host cell proteins, host cell DNA, and transfecting plasmids, and the removal of these impurities is essential to ensure the safety and efficacy of gene therapy, especially to reduce the immunogenicity problems. Our optimization and development of purification processes for adeno-associated viral vectors can help researchers reduce experimental costs and obtain higher purity and titer of AAV viral particles, which can promote the development of gene therapy research.
Explore Our Adeno Associated Virus Vector Purification
Cell culture and infection
Host cells are cultured under suitable conditions and cells are infected with virus solution containing AAV.
Virus collection
After a certain period after infection, the culture fluid containing the virus is collected.
Lysis and clarification
Cells are lysed by sonication or chemical lysing agents, and then cell debris is removed by centrifugation.
Chromatographic purification
Selection of suitable chromatography media and chromatographic separation according to the size and charge characteristics of virus particles.
Virus concentration
Concentration of virus particles by ultrafiltration or centrifugation to increase titer.
Virus detection
Detection of virus purity and titer using enzyme-linked immunosorbent assay (ELISA), real-time quantitative PCR (qPCR) and other methods.
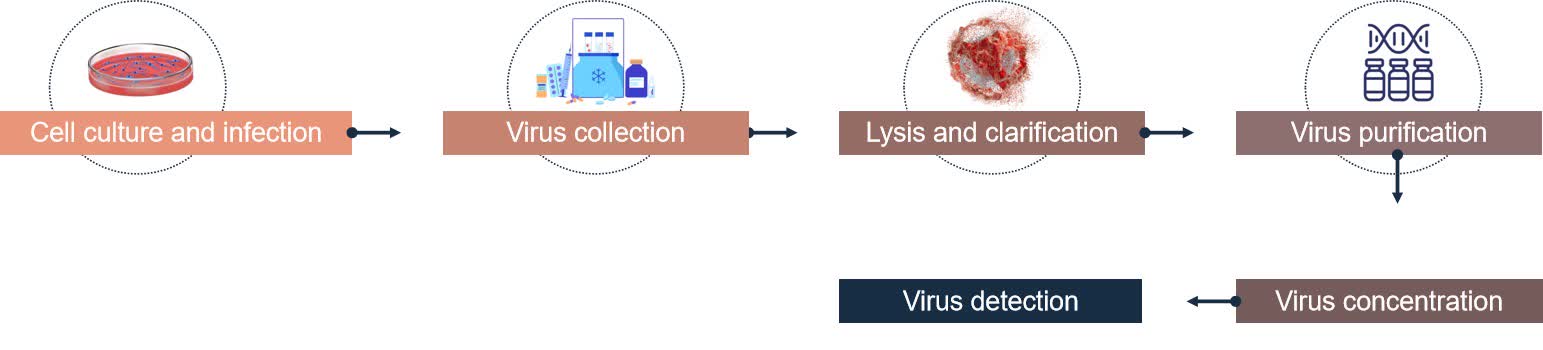 Fig.1 Process of adeno-associated virus vector purification. (CD Formulation)
Fig.1 Process of adeno-associated virus vector purification. (CD Formulation)
Our Solutions for Adeno Associated Virus Vector Purification
In AAV products, residual host cell proteins, bovine serum proteins, nucleic acid enzymes, nucleic acids, and other impurities, not only affect the transfection effect but also cause an immune reaction. To obtain a high-purity product, it is often necessary to combine ultracentrifugation, liquid chromatography, chemical reagents, and ultrafiltration. In the following, we introduce some methods for AAV vector purification.
Ultracentrifugation
Ultracentrifugation is one of the most widely used separation methods for many laboratories, where separation is achieved by centrifugation at high speeds depending on the shape, size, and isodensity points of the impurities and AAV carriers. The advantages of this method are that it is a mature technology that can be operated in general laboratories, it is suitable for all serotypes of carriers, and ultracentrifugation is effective in separating empty shell particles that are very similar in structure.
Liquid chromatography
Currently, chromatography is an important method for AAV purification on a large scale and is also a commonly used method for drug analysis and detection. When the clarified viral liquid flows through the stationary phase, the functional region of the stationary phase interacts with the AAV capsid proteins or the contained genome, and separation is achieved by changing the composition or pH of the mobile phase.
Chemical reagent method
The chemical reagent method is often used in the concentration and precipitation of viral vectors and involves the use of inorganic reagents such as sulfate, calcium phosphate, calcium chloride, or non-ionic surfactants to precipitate the AAV for initial separation.
Purification of AAV vectors plays an important role in ensuring the safety and efficacy of gene therapy, e.g., high-purity AAV vectors can reduce adverse reactions and improve therapeutic efficacy. In addition, the AAV vector purification process is also a key step in the study of viral vectors in gene therapy, which helps researchers to better understand the properties of viral vectors and optimize their application. Therefore, to obtain effective and stable gene introduction results in cultured cells and individual animals, the purity and titer of AAV viral vectors are important influencing factors.
Our Platforms for Adeno Associated Virus Vector Purification
| Platforms & Technologies |
Content Description |
| Affinity capture technology |
We use ligand-specific affinity chromatography packings containing single-domain antibody fragments that bind specifically to a wide range of AAV serotypes, enabling efficient virus capture and impurity removal. |
| Ion exchange purification technology |
We further purify AAV by anion-exchange chromatography, which improves the purity and recovery of AAV through the use of specific packing materials that effectively separate empty and intact capsids by optimized MgCl2 and NaCl concentration gradient elution. |
| Ultrafiltration technology platform |
This is one of our commonly used AAV concentration methods, where AAV particles are trapped by ultrafiltration membranes to achieve effective concentration from the solution while removing small molecule impurities. |
Highlights of Our Adeno Associated Virus Vector Purification
- The purification process has a high yield, low impurity residue, and low production cost.
- Perfect quality control system, the production process is strictly monitored and released.
- Multiple viral vector purification process development, with rich research and service experience.
- Complete equipment configuration, and flexible process route.
Published Data
Technology: AAV purification
Journal: J Vis Exp
Published: 2019
This study describes an efficient and reproducible AAV vector production strategy based on an iodixanol gradient purification strategy. The iodixanol purification method is suitable for obtaining high purity, high titer batches of AAV carriers compared to other purification methods. In addition, the protocol is typically faster than other methods described so far. Additionally, a silver-staining technique for assessing the purity of viral batches and a quantitative polymerase chain reaction (qPCR)-based strategy for quick and accurate vector titer measurement are provided.
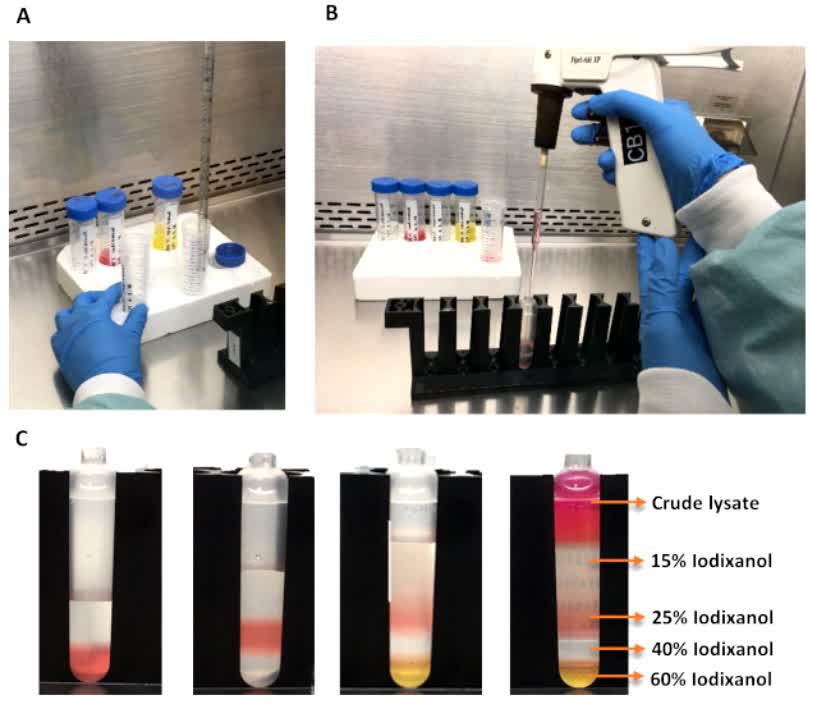 Fig.2 Setup for iodixanol gradient purification and subsequent vector collection. (Fripont S, et al., 2019)
Fig.2 Setup for iodixanol gradient purification and subsequent vector collection. (Fripont S, et al., 2019)
CD Formulation provides reliable technical support and solutions for AAV viral vector purification, and this service plays an important role in the advancement of gene therapy. If you are interested in us, please feel free to contact us.
References
- Fripont S, et al. Production, Purification, and Quality Control for Adeno-associated Virus-based Vectors. J Vis Exp. 2019, 29 (143).
Related Services

 Fig.1 Process of adeno-associated virus vector purification. (CD Formulation)
Fig.1 Process of adeno-associated virus vector purification. (CD Formulation) Fig.2 Setup for iodixanol gradient purification and subsequent vector collection. (Fripont S, et al., 2019)
Fig.2 Setup for iodixanol gradient purification and subsequent vector collection. (Fripont S, et al., 2019)