Non-Viral Gene Delivery Systems Development
Inquiry
Unquestionably, the advancement of gene therapy is aided by the study and creation of non-viral gene delivery technologies. By effectively delivering genetic material to target cells through physical, chemical, or biological means, problems like safety and immunological response that arise from the use of viral vectors can be significantly addressed.
As an industry leader in the creation of dependable, cutting-edge non-viral gene delivery systems, CD Formulation is committed to providing gene delivery system development services. To increase our clients' happiness, we can create non-viral vector development solutions according to the requirements and features of their projects.
Advantages of Non-Viral Gene Delivery Systems Development
- Low side effects. Non-viral gene delivery systems have low immunogenicity and toxicity, which can effectively reduce side effects and safety concerns during use.
- Highly modifiable. Non-viral gene delivery systems are highly modifiable and can adjust vector characteristics to optimize gene delivery efficiency.
- Wide applicability. Non-viral gene delivery systems are suitable for a wide range of nucleic acid types, and are simple and low-cost, making them easy to produce on a large scale.
Explore Our Non-Viral Gene Delivery Systems Development
Non-viral gene delivery technologies are capable of transferring target genes into cells with the aid of a variety of physical, chemical, and biological methods or systems, such as, for example, lipid nanoparticles, bacterial vectors, and exosomes. These systems have promising uses in gene therapy research. Compared to popular viral vector delivery techniques, non-viral gene delivery approaches are safer and less immunogenic. They are useful for reducing immunological reactions and other negative consequences of gene therapy formulations.
We offer non-viral vector development that ensures precision and scalability by optimizing the delivery efficiency and continuously improving the precision of gene editing tools, and we can customize solutions to meet project needs. Our wide range of this service, which involves improving the efficiency of lipid nanoparticles, enhancing the stability of bacterial vectors, and optimizing exosome-based delivery mechanisms, aims to accelerate the process of developing gene therapy agents and provide a safer and more effective solution.
We customize non-viral gene delivery system development
Our Process of Non-Viral Gene Delivery Systems Development
We provide comprehensive and reliable non-viral gene delivery system development services. For different non-viral delivery vectors, we provide customized solutions to ensure that our experimental protocols are more suitable for our customers' needs. The following is our regular development process.
- Target gene selection and optimization. Identification of target genes. Optimize the gene sequence to improve its expression efficiency in the host cell.
- Vector design and preparation. Design the vector structure according to the characteristics of the delivery system. And prepare the vector, which may include synthesizing liposomes, extracting exosomes, or modifying bacteria.
- Gene loading. Efficient loading of the target gene into the vector. Ensure that the binding of the gene to the vector is stable to resist degradation in vivo.
- Vector modification and optimization. Chemical or biological modification of vectors to enhance targeting, penetration, and reduce immune response. Optimize the physical and chemical properties of the vector, such as size, surface charge, and stability, to improve delivery efficiency.
- In vitro validation and evaluation in vivo models. Test the transfection efficiency and gene expression of the vectors in cellular models to assess the cytotoxicity and biocompatibility of the vectors. And further evaluate the delivery efficiency, biodistribution, and safety of the vector in animal models. Finally, we need to adjust the vector design and delivery strategy based on the in vivo results.
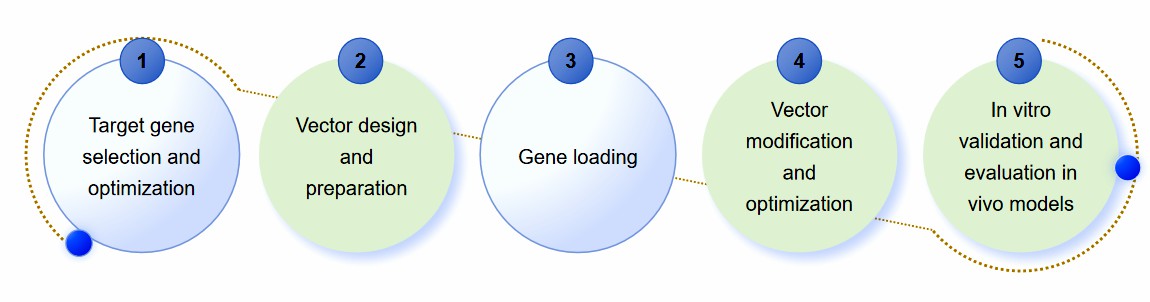 Fig.1 Our development process of non-viral gene delivery systems. (CD Formulation)
Fig.1 Our development process of non-viral gene delivery systems. (CD Formulation)
| Technologies & Platforms |
Content Description |
| CRISPR-Cas9 technology platform |
We integrate target genes into specific parts of non-viral vectors based on CRISPR-Cas9 gene editing methods, such as bacterial vector development services, where we accelerate the development of non-viral gene delivery vectors through the construction of genetically engineered bacteria to facilitate research on the development of gene therapy formulations. |
| RNA interference technology |
RNA interference technology is capable of specifically silencing the expression of target genes. For example, we have developed an innovative solution for bacteria-mediated RNA interference by utilizing bacteria as vectors to deliver therapeutic RNA to target cells. |
| Lipid nanoparticle (LNP) technology platform |
The components in the LNP delivery system include phospholipids, cholesterol, and polyethylene glycol (PEG), which make LNP biocompatible and low-toxicity in living organisms. The LNP delivery method offers a dependable alternative for the production of gene therapy formulations and is successful in enhancing the targeting, stability, and delivery efficiency of gene therapy formulations. |
Highlights of Our Non-Viral Gene Delivery Systems Development
- Efficiency and safety. We continually optimize and streamline our development processes and utilize state-of-the-art technologies to continuously improve productivity, reduce potential risks, and provide reliable research support to our customers.
- Customized solutions. We provide customized solutions for our clients, focusing on the development of non-viral vectors to ensure that all types of research objectives are met.
- Dedicated research team. Our experts and technical team have extensive expertise and years of service experience in non-viral vector development, enabling precise and efficient target gene delivery to target cells.
- Innovative solutions. We are committed to providing innovative non-viral gene delivery development services to improve the success of gene expression and help researchers advance progress in the development of gene therapy formulations.
Published Data
Technology: Non-viral gene delivery technology
Journal: Front Bioeng Biotechnol
IF: 5.7
Published: 2020
Results: Non-viral gene delivery methods are more suitable for translational can deliver genes more safely and can transiently regulate gene expression in vivo and in vitro. This article summarizes recent advances in the field of orthopaedics, focusing on the bones and joints of the musculoskeletal system, and the authors discuss methods for the delivery of micro RNA (miRNA) and silencing RNA (siRNA) and DNA plasmids by providing a brief overview of existing techniques such as the use of nanospheres, engineered vesicles, lipid transfection, and in vivo electroporation.
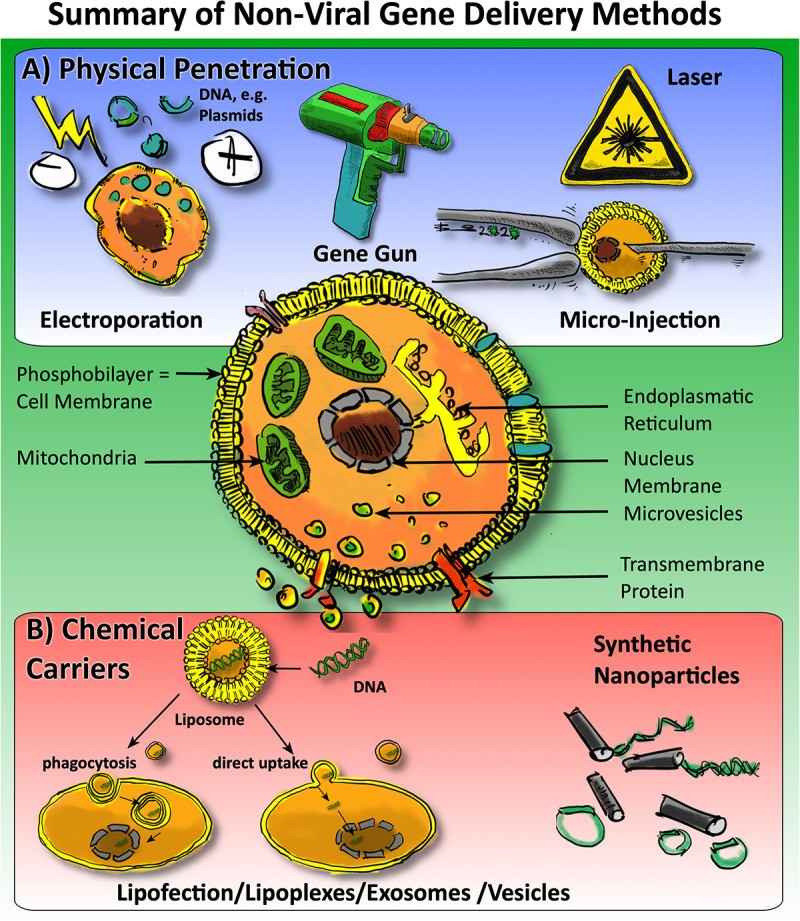 Fig.2 Non-viral approaches for gene delivery to cells in orthopedics. (Gantenbein B, et al., 2020)
Fig.2 Non-viral approaches for gene delivery to cells in orthopedics. (Gantenbein B, et al., 2020)
CD Formulation provides comprehensive and reliable development services in gene therapy formulation development. We offer development of non-viral gene delivery systems that provide our clients with a solid foundation for gene therapy formulation development and ensure the success of their projects. If you are interested in us, please feel free to contact us.
References
- Gantenbein B, et al. Non-viral Gene Delivery Methods for Bone and Joints. Front Bioeng Biotechnol. 2020, 8:598466.
Related Services

 Fig.1 Our development process of non-viral gene delivery systems. (CD Formulation)
Fig.1 Our development process of non-viral gene delivery systems. (CD Formulation) Fig.2 Non-viral approaches for gene delivery to cells in orthopedics. (Gantenbein B, et al., 2020)
Fig.2 Non-viral approaches for gene delivery to cells in orthopedics. (Gantenbein B, et al., 2020)