Naked DNA Development Service
Inquiry
Naked DNA-mediated gene therapy refers to the delivery of naked DNA (without viral vectors) carrying target genes directly to the target cells to realize the transcription and expression of genes with the help of the cell's own genetic expression mechanism, so as to achieve the purpose of preventing or treating diseases. This method does not need to rely on viral vectors, so it is highly safe and simple. Naked DNA is injected directly into target cells by intramuscular injection, intradermal injection or intravenous injection, and the expression of target genes is accomplished in the cells, thus producing therapeutic effects. This approach can be applied to gene therapy for a variety of diseases, such as hereditary diseases, tumors, infectious diseases, and so on.
CD Formulation is a leading global biotechnology service provider, with many years of professional and rich experience, we can provide you with a full range of gene therapy vector delivery system development services. We can provide customized gene therapy non-viral vector development services according to your project requirements, and our professional knowledge and advanced technology can effectively help you promote the research and development of gene therapy.
Advantages of Naked DNA Development
- Low immunogenicity. Naked DNA itself is of low immunogenicity and will not trigger a strong immune response. It can be administered repeatedly without producing neutralizing antibodies.
- High safety. Compared with viral vector gene therapy, naked DNA therapy does not have the risk of viral infection and avoids potential safety hazards such as viral replication and immune response, thus it has higher safety.
- Simple preparation. Naked DNA does not require complex preparation of viral vectors, the preparation process is relatively simple and fast, low cost, and easy to mass production, which makes gene therapy more economically feasible.
- Strong targeting. By regulating the DNA sequence and the route of administration, it can realize efficient target transfection of specific cells or tissues.
- Long-lasting gene expression. Naked DNA can be continuously transcribed and expressed in the cell nucleus, which can realize long-term therapeutic effects.
What We Offer to Develop Naked DNA?
Screening of target genes and vector construction
First of all, we need to determine the therapeutic target and select suitable therapeutic genes, such as corrective genes, suicide genes or immunomodulatory genes, etc. Then we will clone the target gene into the appropriate plasmid vector and construct the expression vector. Then, the target gene should be cloned into the appropriate plasmid vector and the expression vector should be constructed.
Purification and preparation of naked DNA
Plasmid amplification, extraction, purification and other technical means are used to obtain high-purity naked DNA samples. The concentration, purity and structural integrity of naked DNA should be strictly controlled to ensure the quality.
Optimization of route of administration and dosage
According to the different indications, the appropriate route of administration, such as intramuscular injection, intradermal injection, intravenous injection, etc., should be selected. At the same time, the dosage should be optimized to ensure effective and safe treatment.
Transfection and gene expression in target cells
After the naked DNA enters the target cells, it can realize the expression of the target gene through the transcription and translation mechanism in the cells. The transfection efficiency can be evaluated by monitoring the gene expression level and therapeutic effect.
Applications of Our Naked DNA Development Service
- Hereditary diseases. Naked DNA can be involved in immunoregulation, apoptosis, or other processes that promote tumor cell apoptosis or stimulate the immune system to prevent tumor growth.
- Tumor diseases. Naked DNA vectors express genes involved in immunoregulation, apoptosis, or other processes that promote tumor cell apoptosis or stimulate the immune system to prevent tumor growth.
- Infectious diseases. To prevent and treat infectious diseases including AIDS, malaria, tuberculosis, and others, the body is stimulated to create an immune response against pathogens through the production of vaccine antigen genes or immunoregulatory genes.
- Other diseases. Naked DNA gene therapy can also be applied to the treatment of cardiovascular diseases, neurological diseases, metabolic diseases and so on. For example, it can be used to treat vascular diseases by expressing the vascular growth factor gene and to treat neurological diseases by expressing the nerve growth factor gene.
Our Platforms for Naked DNA Development
| Technologies & Platforms |
Content Description |
| Naked DNA efficient transfection technology platform |
Our researchers have developed several technology platforms for different cell types and therapeutic targets. Electroshock transfection, liposome transfection and bioluminescence transfection are among the commonly used technology platforms. Electroshock transfection is achieved by applying electrical pulses to induce naked DNA to enter the cell, liposome transfection is achieved by using synthetic liposomes to wrap the DNA and disrupt the cell membrane, and bioluminescent transfection utilizes a complex of specific proteins and genes to introduce the DNA into the cell, and at the same time, generates fluorescent signals to monitor the efficiency of the transfection. |
| In vivo gene editing technology platform |
In vivo gene editing therapies require efficient and safe gene delivery systems to deliver therapeutic genes to relevant organs and tissues in the human body. Currently, we offer several major in vivo gene editing delivery technologies such as lipid nanoparticle (LNP) delivery and virus-like particle (VLP) delivery for non-viral vector delivery. |
| Hydrodynamic retrograde intrabiliary injection (HRII) technology platform |
The intrabiliary injection approach has the potential for injection of vector DNA by ERCP-guided hydrodynamic delivery in combination with sequential multilobular injections, which opens new avenues for direct gene delivery to the liver. |
| Zinc finger nuclease (ZFN)technology platform |
This technology platform is used to recognize and bind specific DNA sequence repeats consisting of zinc finger proteins and nuclease structural domains of restriction endonucleases that non-specifically cleave DNA by dimerization. |
Highlights of Our Naked DNA Development Service
- Customized solutions. We provide customized solutions for non-viral vector development based on our customers' research goals. This technical service ensures that naked DNA is optimized for your specific application.
- Professional research team. Our team has deep knowledge in the field of gene delivery, which enables us to provide the most effective and reliable technical methods to deliver naked DNA to target cells.
- Innovative solutions. We are always at the forefront of gene therapy development. We provide innovative naked DNA development technologies to increase the possibility of successful gene expression.
- Efficiency and safety. We continue to simplify the development process to make naked DNA more efficient and safer in gene delivery.
Published Data
Technology: Hydrodynamic retrograde intrabiliary injection (HRII) technology
Journal: Mol Ther Methods Clin Dev.
IF: 4.7
Published: 2022
Results: The study aimed to improve hepatic gene therapy in newborns by targeting periportal hepatocytes in weaned pigs. The researchers established a method for delivering non-integrating naked DNA vectors using hydrodynamic retrograde intrabiliary injection (HRII). The procedure involved a surgical approach with laparotomy and temporary liver isolation. A catheter was inserted into the common bile duct through enterotomy to deliver the vectors. The delivery was performed under optimal conditions to avoid histological abnormalities in liver tissue. The HRII method, while less efficient than intraportal delivery, presents a less invasive and potentially more practical approach for hepatic gene therapy in newborns, with the added benefit of being less distressful for the subjects and allowing for repeat treatments.
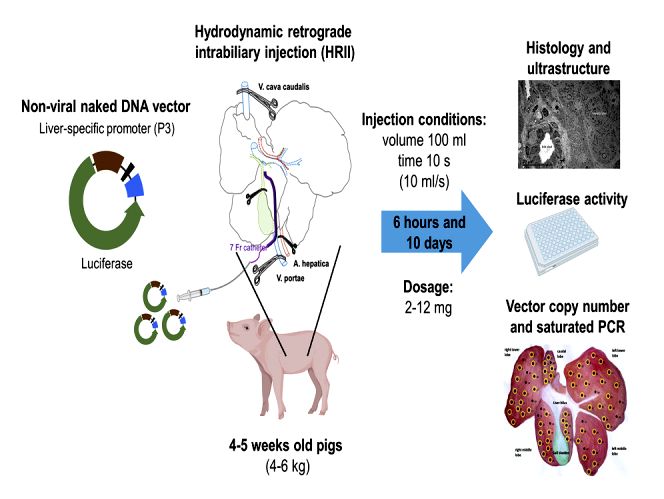 Fig.1 Direct intrabiliary injection of naked DNA vectors in a model system with newborn piglets. (Chan T, et al., 2022)
Fig.1 Direct intrabiliary injection of naked DNA vectors in a model system with newborn piglets. (Chan T, et al., 2022)
CD Formulation has many years of research service experience in gene therapy. In naked DNA development, we have established core technologies including target gene design and screening, plasmid vector construction, production processes, quality standards, and so on. Relying on our existing technology platform, we are committed to promoting the research and application of naked DNA delivery system in gene therapy. If you are interested in us, please feel free to contact us.
Reference
- Chan T, Grisch-Chan HM, et al. Delivery of non-viral naked DNA vectors to liver in small weaned pigs by hydrodynamic retrograde intrabiliary injection. Mol Ther Methods Clin Dev. 2022, 24:268-279.
Related Services

 Fig.1 Direct intrabiliary injection of naked DNA vectors in a model system with newborn piglets. (Chan T, et al., 2022)
Fig.1 Direct intrabiliary injection of naked DNA vectors in a model system with newborn piglets. (Chan T, et al., 2022)