Exosome Delivery Vector Development Service
Inquiry
Exosomes are nanoscale lipid bilayer vesicles actively secreted by cells, with a variety of biological components, such as proteins, lipids, and nucleic acids, encapsulated in their lumen or lipid bilayer. Exosomes have low immunogenicity, high physicochemical stability, high tissue penetration, and innate transport ability, and thus are expected to be a novel class of drug delivery carriers. It has been found that exosomes play a crucial role in long-distance intercellular communication because they can reach other cells and tissues through the circulatory system to produce long-range regulatory effects. Thus, exosomes are natural carriers of intercellular communication. This function then induces researchers to develop exosome-based drug delivery systems, especially using exosomes in gene therapy applications. CD Formulation provides exosome delivery vector development, and we can develop customized exosome delivery vectors according to our clients' project needs, to achieve the effective promotion of purposeful non-viral delivery systems for research and application in gene therapy diseases.
Advantages of Exosome Delivery Vectors Development
Exosomes have been used as non-viral vectors for gene delivery and have shown high biocompatibility, low clearance and suitability for cell-targeted delivery.
- Greater stability. Exhibits enhanced stability in the bloodstream, allowing exosomes to be transported over long distances in vivo under both physiological and pathological conditions.
- Ability to encapsulate soluble drugs. Liposomes are less efficient at packaging hydrophilic substances and they are limited in delivering nucleic acids. Exosomes have a hydrophilic core, enabling them to package soluble drugs.
- It can cross a wide range of biological barriers. Nanoscale stature allows liposomes to cross a variety of biological barriers and selectively penetrate lesions such as tumors or inflamed tissues through enhanced permeability and retention effects. In addition, exosomes can remain there for a long time after reaching the target tissue.
- Multifunctional vectors. Exosomes can encapsulate and deliver various biological cargoes, such as small RNAs, mRNAs, and proteins.
What We Offer to Develop Exosome Delivery Vectors?
Exosomes as vectors for CRISPR-Cas9 plasmids
In contrast to viral vectors, exosomes from certain cellular sources can selectively deliver cargo to tumor tissues. For example, it was found that exosomes of cancer cell origin can translocate the CRISPR-Cas9 system to target tumors.
Engineered exosomes for CRISPR-Cas9 delivery
Natural exosomes are not designed to deliver large molecules, such as plasmids. As a result, the load of plasmids in exosomes is low. Exosomes then need to be engineered to increase the efficiency and capacity of the plasmid. Engineered exosomes also endow these transporter nanoparticles with the ability to target cargo to specific cells or tissues.
Exosome-liposome hybrids for gene delivery
The nanoscale size of exosomes (40-160 nm) makes them competent carriers for small therapeutic agents such as compounds, siRNAs, and miRNAs. For large molecules, such as CRISPR-Cas9 expression plasmids with a minimum size of 5-6 kb, the capacity of natural exosomes remains relatively low. On the other hand, liposomes are capable of encapsulating and delivering large plasmids but are highly cytotoxic due to the unnatural nature of lipids. The fusion of the lipid bilayer of the exosome membrane with liposomes forms exosome-liposome heterodimers, which allow for the encapsulation and delivery of large DNA molecules, such as CRISPR-Cas9 expression plasmids, and also alleviate the toxicity problem of liposomes.
Applications of Our Exosome Delivery Vectors Development Service
- Genetic disease treatment. Genetic illnesses including cystic fibrosis and inherited blindness can be treated with exosomes. By delivering functional genes, exosomes can fix damaged genes or genomic areas in the patient's body. To help the patient regain normal physiological activities, these repaired genes may comprise normal copies, mutation corrections, gene additions, etc.
- Cancer therapy. Exosomes have potential applications in cancer therapy. On the one hand, exosomes can be used for gene expression-targeted therapy, such as delivering mitogen suppression genes (Tumor Suppressor Genes) to inhibit the proliferative ability of cancer cells or delivering interfering RNAs (siRNAs) that inhibit activated oncogenes. On the other hand, exosomes can also carry immunomodulatory and anti-tumor genes to enhance the effectiveness of immunotherapy in patients.
- Neurodegenerative disease therapy. Exosomes also have potential in the treatment of neurodegenerative diseases. For example, exosomes can promote the growth and connectivity of nerve cells in patients by carrying neurofactor genes such as brain-derived neurotrophic factor (BDNF) or nerve growth factor (NGF). This has important implications for the treatment of diseases such as Parkinson's disease and Alzheimer's disease.
Our Platforms for Exosome Delivery Vectors Development
| Technologies & Platforms |
Content Description |
| CRISPR-Cas9 technology platform |
Guided by CRISPR sequences, Cas9 enzymes can edit genomic DNA in cells with unprecedented precision, efficiency, and flexibility. We offer a CRSPR-Cas9 technology platform that can be realized through physical interactions, chemical modifications, and biological vectors when enabling exosome-mediated delivery of the CRISPR-Cas9 system. |
| Cellular nanopore biochip technology platform |
Cells were transfected with plasmid DNA and the transfected cells were stimulated by local and transient electrical stimulation. This nanopiercing procedure facilitated the release of exosomes carrying transcribed mRNA and targeted peptides from the cells, resulting in a multifold increase in exosomes and a high increase in exosomal mRNA transcription products. |
| Exosome purification technology platform |
After completing the production, we need to purify the exosomes from the other components of the culture medium. Exosome purification is performed using our purification technology platform, e.g. by centrifugation. |
| Cas9 ribonucleoprotein (RNP) technology platform |
The Cas-gRNA complex is known as a ribonucleoprotein (RNP). Several methods have been developed for direct delivery of RNPs to cells. Frequently, RNP is delivered into cells in culture by lipofection or electroporation.RNPs are usually electroporated using a nucleofection protocol because this allows them to quickly enter cells and begin cutting genomes. |
Highlights of Our Exosome Delivery Vectors Development Service
- Multiple cooperative units. Our company has established a mature technology platform for exosome vector development, and we have established cooperation with many key hospitals to jointly promote the vigorous development of exosome vector development in gene therapy.
- Advanced technology platforms. Based on our proprietary intellectual property platform, we have developed an advanced technology platform for exosome vector development. In addition, we continue to introduce efficient research tools for exosome development centered on customer needs.
- High competitiveness. Years of honing and building up our technology have given us a core competitive exosome vector development platform. The company's future goals will still be to produce exosome vectors for gene therapy, thoroughly develop the underlying technology, and provide top-notch exosome vector development services.
Published Data
Technology: Cas9 ribonucleoprotein (RNP) technology
Journal: Sci Adv.
IF: 13.6
Published: 2022
Results: CRISPR-Cas9 gene editing has emerged as a powerful therapeutic technology, but the lack of safe and efficient in vivo delivery systems, especially for tissue-specific vectors, limits its broad clinical applications. Delivery of Cas9 ribonucleoprotein (RNP) owns competitive advantages over other options. However, the large size of RNPs exceeds the loading capacity of currently available delivery vectors. This study report a previously unidentified genome editing delivery system, named exosomeRNP, in which Cas9 RNPs were loaded into purified exosomes isolated from hepatic stellate cells through electroporation. ExosomeRNP facilitated effective cytosolic delivery of RNP in vitro while specifically accumulated in the liver tissue in vivo. ExosomeRNP showed vigorous therapeutic potential in acute liver injury, chronic liver fibrosis, and hepatocellular carcinoma mouse models via targeting p53 up-regulated modulator of apoptosis (PUMA), cyclin E1 (CcnE1), and K (lysine) acetyltransferase 5 (KAT5), respectively. The developed exosomeRNP provides a feasible platform for precise and tissue-specific gene therapies for liver diseases.
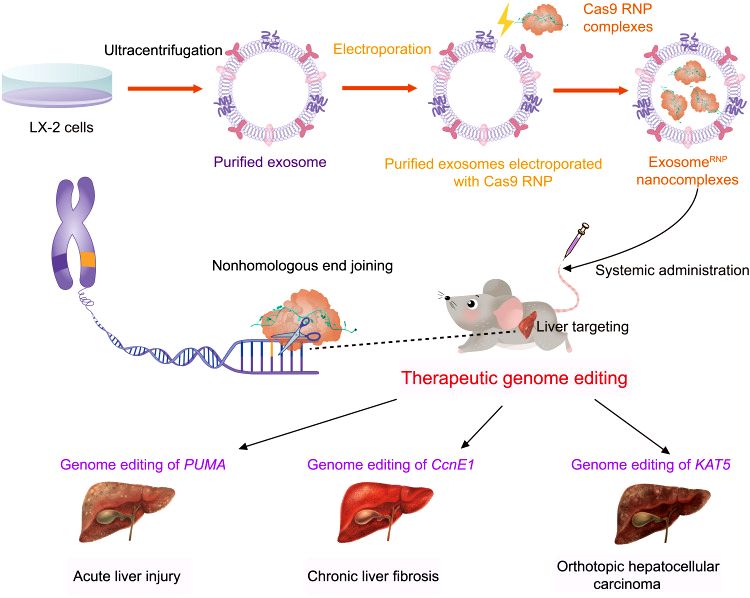 Fig.1 Schematic illustration of exosome for in vivo delivery of Cas9 RNP for the treatment of liver disorders. (Wan T, et al. 2022)
Fig.1 Schematic illustration of exosome for in vivo delivery of Cas9 RNP for the treatment of liver disorders. (Wan T, et al. 2022)
CD Formulation designs exosomal vectors for specific delivery of genes that can provide effective support for gene therapy. We develop exosomal vectors that can mediate the delivery of various gene therapy molecules or vectors, and we play an important role in helping researchers advance the study and application of non-viral vectors for gene delivery. If you are interested in us, please feel free to contact us.
Reference
- Wan T, Zhong J, et al. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci Adv. 2022, 8(37):eabp9435.
Related Services

 Fig.1 Schematic illustration of exosome for in vivo delivery of Cas9 RNP for the treatment of liver disorders. (Wan T, et al. 2022)
Fig.1 Schematic illustration of exosome for in vivo delivery of Cas9 RNP for the treatment of liver disorders. (Wan T, et al. 2022)