Stem Cell Vehicle Development
Inquiry
The use of stem cells as vehicles for gene therapy is a new technology that maximizes the therapeutic effects of stem cells by engineering them to express therapeutic genes for the target disease. CD Formulation has an advanced and reliable stem cell development technology platform that provides researchers with a wide range of modified stem cells that can effectively deliver therapeutic genes, thereby advancing gene therapy research.
Types of Stem Cells We Develop
- Mesenchymal Stem Cells (MSC). We develop and design mesenchymal stem cell (MSC) vehicles that can be genetically engineered to introduce beneficial genes to replace abnormal gene function at the tissue and organ level. The range of diseases for which MSCs can provide therapeutic benefit may be greatly broadened by using gene therapy to engineer MSCs to increase the natural production of specific desired proteins or to enable them to express proteins outside of their natural pool. We offer various types of genetically modified MSCs for gene therapy research to enhance their natural ability to mediate repair in multiple tissues.
- Neural Stem Cells (NSC). NSCs migrate to designated sites of cellular injury to repair or replace dead neuronal cells. NSCs undergo a process of isolation, proliferation, genetic manipulation, and differentiation in vitro before being reintroduced into the developmentally or pathologically altered central nervous system. We have developed neural stem cell vehicles to efficiently introduce therapeutic genes into adult neural stem cells, including adenoviral and VSV-G pseudotyped retroviral vectors.
Advantages of Stem Cell Vehicle
- Tumor tropism. NSCs and MSC, have the property of naturally migrating toward the tumor site, which allows them to be used as drug delivery vehicles to deliver therapeutic agents directly to the tumor site.
- Low immunogenicity. Certain stem cell types, particularly MSC, have low immunogenicity, which reduces the risk of them triggering an immune rejection reaction during treatment.
- Targeted delivery of genes. Stem cells can be used as vehicles of genes for targeted delivery to specific cells or tissues, thereby increasing the precision of treatment and reducing side effects.
- Safety and long-term expression. Stem cell vectors offer improved safety and long-term transgene expression in gene therapy diseases.
Our Services for Developing Stem Cell Vehicle
Stem cells selection and culture
Selection of suitable stem cell types, such as neural stem cells (NSCs), mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs) and so on. For example, human adipose-derived mesenchymal stem cells (MSCs) are derived from adipose tissue that is extracted from MSCs. The extracted stem cells are first cultured in vitro in primary cultures, usually using specific media and under conditions that support the adherent growth of stem cells. To obtain a sufficient number of stem cells for research or therapeutic use, we need to perform cell expansion in vitro. After this process is completed, we can genetically modify them to have drug delivery capability according to the client's project needs.
vehicles design
In stem cell gene delivery vector development, we aim to design vehicles that are capable of efficiently delivering drugs or gene editing tools. This includes viral vectors (e.g., adeno-associated virus, lentivirus, adenovirus) and non-viral vectors (e.g., lipid nanoparticles, virus-like particles).
Viral vehicles transduction
Viral vectors, such as lentiviruses, adenoviruses, or retroviruses, can efficiently deliver nucleic acids into MSCs. These viral vectors are modified to remove pathogenicity but retain the ability to infect cells.
Non-viral transfection
These include liposome-mediated transfection, electroporation and nanoparticle-mediated transfection. These methods do not rely on viruses but deliver nucleic acids directly into cells by physical or chemical means.
Optimizing Stem Cell Vehicle for Gene Therapy
- Cancer treatments. MSC can selectively target sites of injury or inflammation, and then mediate repair of the injured tissue by implanting and generating tissue-specific cells, and promote healing by releasing trophic factors to inhibit the inflammatory response and activating the endogenous repair mechanisms of the tissue. MSCs are not only indicated for solid tumors but also have great therapeutic value in the treatment of glioblastoma multiforme (GBM) and lung metastases caused by breast cancer and melanoma. Has tremendous therapeutic value.
- Diabetes treatment. It was found that human adipose-derived MSCs were genetically modified with Exendin-4 using a lentiviral infection system to enhance the efficacy of MSCs against type 2 diabetes mellitus (T2DM). Through genetic modification, MSCs were able to achieve long-lasting release of Exendin-4 and enhance their survival and efficacy in diabetic hyperglycemic environments through autocrine effects, while secreting other active factors that could protect pancreatic β-cells through endocytosis and improve insulin sensitivity in hepatocytes and reduce lipid accumulation in hepatocytes through paracrine effects. These results suggest that MSCs have great potential as vehicles of gene therapy in providing new therapeutic approaches and improving existing treatment strategies.
- Neuronal repair. Their differentiation potential and genetic plasticity make them a preferred modality for cell transplantation. Neural stem cells (NSC) are the primary vehicles for gene and molecular therapies in the CNS and have been used to treat stroke, Parkinson's disease, Huntington's disease, and multiple sclerosis.
Our Platforms for Stem Cell Vehicle Development
| Platforms & Technologies |
Content Description |
| Homing of stem cells |
Homing enhances repair mechanisms and increases cellular inventory and signaling molecules. We work to optimize cell viability, migration, targeting and tracking to enhance the potential for stem cell homing. |
| Gene editing technology |
We have established gene editing technology platforms, for example, through systems such as CRISPR-Cas9, whereby stem cells are genetically modified to enhance their therapeutic potential through precise gene editing of target genes. |
| Gene targeting technology |
We have established a gene-targeting technology platform that works by introducing therapeutic genes into stem cells and then safely delivering the genetically modified cells back to the patient. |
Highlights of Our Stem Cell Vehicle Development
- Advanced gene editing technology. We have adopted cutting-edge gene editing technologies such as CRISPR/Cas9 to ensure the precision and efficiency of gene modification.
- Versatile stem cell platform. We have a variety of advanced stem cell platforms designed to enhance the safety and totipotency of stem cells, as well as the accuracy and editing efficiency of gene editing.
- Advanced algorithmic guidance. We also utilize computer biology algorithms and single-cell multi-omics analyses, which are used to significantly improve the efficiency and functionality of stem cell expansion and differentiation.
- Quality control and management systems. We have established a stable and controlled stem cell vector development system as well as a comprehensive quality management system to ensure the safety and targeting of our stem cell vectors.
Published Data
Technology: Homing of stem cells
Journal: Expert Opin Drug Deliv
IF: 6.6
Published: 2021
This study discusses the origin and inter-donor heterogeneity of MSC, their tumor-homing mechanisms, and the route of drug delivery of MSC in MSC-based cancer therapy. We then summarize the therapeutic applications of MSC as drug delivery vehicles for therapeutic genes or anticancer drugs and the mechanisms of drug delivery from drug-loaded MSC to cancer cells.
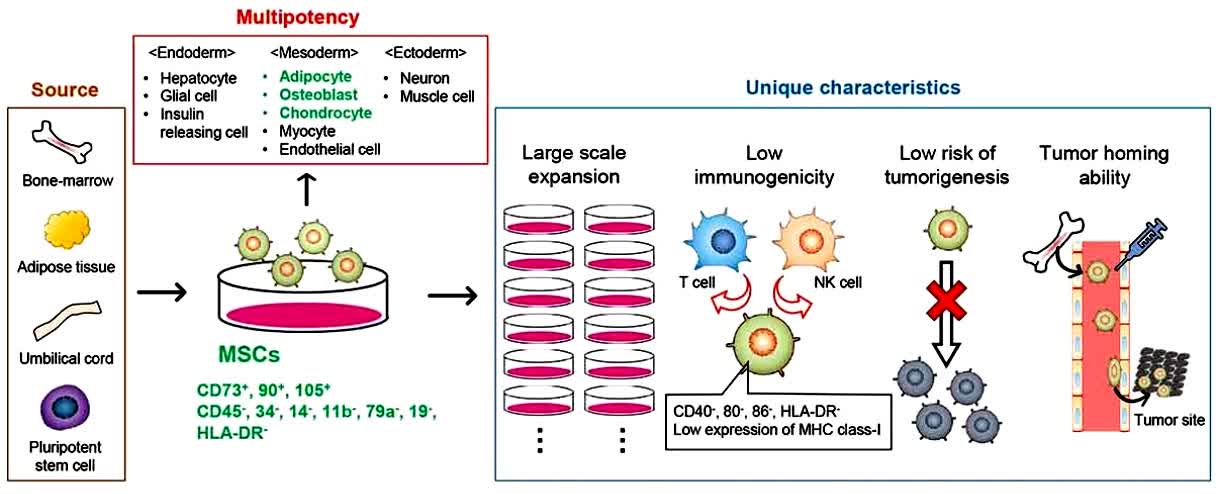 Fig.2 The properties and therapeutic applications of mesenchymal stem cells. (Takayama Y, et al., 2021)
Fig.2 The properties and therapeutic applications of mesenchymal stem cells. (Takayama Y, et al., 2021)
CD Formulation has many years of experience in providing stem cell services, our professional research team will provide customized stem cell vector development solutions according to your project requirements, and we sincerely look forward to working with you. If you are interested in us, please feel free to contact us.
References
- Takayama Y, et al. Mesenchymal stem/stromal cells as next-generation drug delivery vehicles for cancer therapeutics. Expert Opin Drug Deliv. 2021 Nov;18(11):1627-1642.
Related Services


 Fig.2 The properties and therapeutic applications of mesenchymal stem cells. (Takayama Y, et al., 2021)
Fig.2 The properties and therapeutic applications of mesenchymal stem cells. (Takayama Y, et al., 2021)