Gene Therapy Formulation Preclinical Research Services
Inquiry
As a key bridge between laboratory breakthroughs and patient treatments, preclinical studies of gene therapy formulations bear a triple mission, i.e., to validate the biosafety of the therapeutic vectors, to assess the precision of gene editing, and to confirm the durability of the therapeutic effects. CD Formulation provides comprehensive preclinical service support for the development of gene therapy formulations, aiming to help researchers accelerate the development of gene therapy formulations for research.
Importance of Gene Therapy Formulation Preclinical Research Services
- Assessment of safety. Preclinical studies of gene therapy agents are conducted to further confirm the safety of gene therapy agents through toxicity assessment, gene insertion risk, and long-term risk assessment.
- Validate effectiveness. Preclinical studies verify the therapeutic effect of gene therapy agents through various experiments, providing a scientific basis for clinical trials.
- Optimize drug design. Preclinical studies provide important data support for the design and optimization of gene therapy formulations, including carrier selection and optimization, dose optimization, and route administration optimization.
Explore Our Gene Therapy Formulation Preclinical Research Services
Relying on our mature gene therapy drug development platform, we have been deeply engaged in preclinical research for many years, and provide a full-process support system for pharmacological activity evaluation, biodistribution characterization, and safety validation for the development of innovative gene therapy agents.
Gene Therapy Formulation Toxicology Service
As a key link before entering clinical trials, we have established a complete toxicology research matrix. By systematically conducting modular studies such as conventional toxicity testing, immunogenicity or toxicity testing, genotoxicity screening, and reproductive toxicity assessment, we can deeply analyze the potential risk characteristics of gene therapy formulations and provide scientific and rigorous data support for the design of clinical research protocols.
Gene Therapy Formulation Pharmacology Service
Based on the perspective of translational medicine, our research team focuses on exploring the core mechanism of gene therapy agents. We provide pharmacological analysis of gene therapy agents through multi-dimensional mechanism studies, ranging from the visualization of vector delivery pathway tracking to the analysis of target tissue localization efficiency, from the monitoring of gene expression kinetics to the verification of potential off-target effects.
Gene Therapy Formulation Pharmacokinetics Service
We provide high-quality pharmacokinetic services for the special metabolic pattern of gene therapy drugs. Through innovative testing technology, we can dynamically monitor the distribution of drugs in plasma and tissue samples, combined with physiological pharmacokinetic (PBPK) modeling and other advanced analytical tools, we can systematically analyze the characteristics of the whole process of drug absorption, transport, metabolism and clearance, and provide key decision-making basis for dosage optimization and dosing regimen design.
Gene Therapy Formulation Pharmacodynamic Assay
We assessed the efficacy of gene therapy formulations by monitoring the expression levels of biomarkers. Gene expression and protein activity are evaluated, gene therapy formulations are tested for immunogenicity and analyzed for possible off-target effects and safety, and we conduct in vivo efficacy trials to study the efficacy of gene therapy formulations.
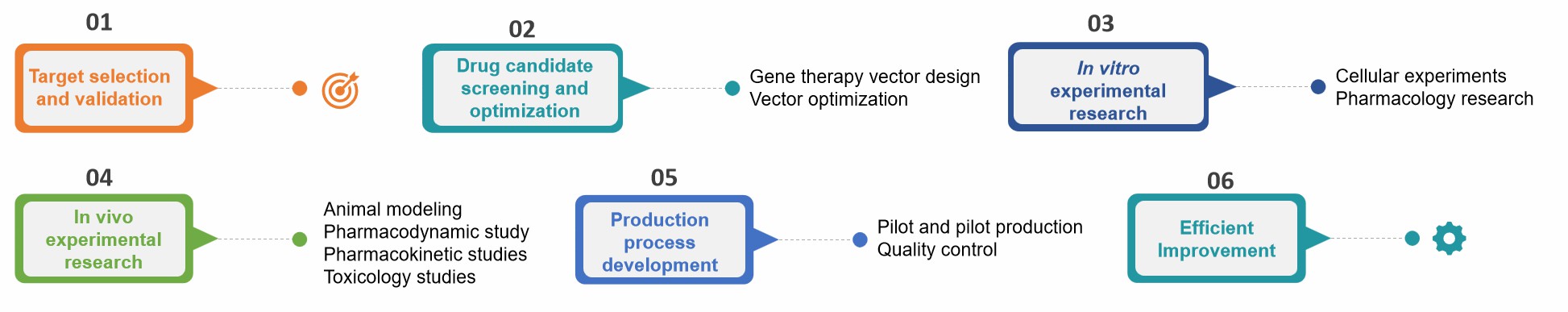 Fig.2 Our process of gene therapy formulation preclinical research. (CD Formulation)
Fig.2 Our process of gene therapy formulation preclinical research. (CD Formulation)
Our Platforms for Gene Therapy Formulation Preclinical Research Services
| Technologies & Platforms |
Content |
| Nucleic acid level analysis platform |
qPCR, RT-qPCR, dPCR |
| Cell level analysis platform |
Cell-counter, FACS, ELISPOT, TCID50 |
| Protein level analysis platform |
ELISA, FIA, ECL, RIA |
| Advanced instrumentation |
Flow cytometer, digital PCR instrument, electrochemiluminescence analyzer, and so on. |
Highlights of Our Gene Therapy Formulation Preclinical Research Services
- One-stop gene therapy formulation development service. We provide one-stop gene therapy formulation development services, including viral vector construction and development, viral packaging and production, disease model construction, to gene therapy formulation effectiveness evaluation.
- Mature biosignature platform. We have a composite background team that has accumulated a large amount of bioinformatics and gene editing data, which greatly facilitates gene therapy formulation development and research.
- Abundant and high-quality animal models. We have abundant animal model resources, which can be better used for preclinical research and the development of gene therapy formulations.
- Customized research protocols. Our team of technicians has rich experience and expertise in designing and executing various in vitro experiments according to customers' needs.
CD Formulation is an industry leader in the development of gene therapy formulations, and our preclinical research services bring technical support to accelerate the development of gene therapy formulations. If you are interested in us, please feel free to contact us.
Related Services


 Fig.2 Our process of gene therapy formulation preclinical research. (CD Formulation)
Fig.2 Our process of gene therapy formulation preclinical research. (CD Formulation) 