Gene Therapy Formulation Toxicology Services
Inquiry
The development of gene therapy formulations offers potential cures for genetic diseases, tumors, and rare diseases. However, its complex mechanism of action may give rise to unique toxicity risks, such as vector-associated immunogenicity, off-target effects of genes, and uncontrollability of long-term expression products. As a pioneer in the development of gene therapy formulations, CD Formulation provides international standard gene therapy toxicology services to help clients systematically assess the safety of their drug candidates based on decades of industry experience and scientific expertise.
Importance of Gene Therapy Formulation Toxicology Services
- Guiding experimental design. The data structure of toxicology studies can provide data support for the design of experiments for the development of subsequent gene therapy formulations, and further help researchers to identify potential toxicity reactions and biomarkers.
- Reduce development risk. Toxicology studies can identify potential toxicity issues early in the development of gene therapy formulations, avoiding failures due to safety issues in subsequent trials, and can effectively help reduce development costs and time.
- Support regulatory approval. Toxicology study data is an important basis for regulatory approval of gene therapy formulations.
Explore Our Gene Therapy Formulation Toxicology Services
Our extensive experience in toxicology research for gene therapy formulation development allows us to provide comprehensive toxicology research service support for gene therapy product development.
We can help our clients with general toxicity testing of gene therapy formulations to ensure their safety. Some of the general toxicity testing of gene therapy formulations we cover are acute toxicity assessment, subchronic and chronic toxicity assessment, pharmacokinetic and biodistribution characterization, genotoxicity, and carcinogenicity assessment.
Accurate quantification and assessment of genotoxicity are integral to ensuring the reliability and efficacy of gene therapy formulation development. Genotoxicity assays for gene therapy formulations can effectively and accurately predict the risks that may arise after administration, thereby optimizing formulation design to minimize adverse effects. We provide genotoxicity assessments, including mutagenicity, chromosome damage, insertion mutagenicity, and long-term genomic stability assessments.
We provide immunotoxicity testing services for gene therapy formulations to further ensure the safety and compliance of gene therapy products. Our assessments include viral vector antibody generation, cellular immune response, cytokine storm risk, and unintended immune effects to minimize adverse effects caused by gene therapy formulations.
In addition, we offer other toxicology evaluations.
Single and repeated administration toxicity tests. This service covers rodent and non-rodent animal models and is designed to assess the acute toxicity and chronic toxicity of gene therapy formulations at different doses.
Toxicokinetic studies. We offer this service to analyze the distribution, metabolism, and excretion of gene therapy products in the body.
Specialized toxicity testing. Studies for this service include localized toxicity, such as hemolysis, allergy, irritation tests, and carcinogenicity studies.
We also offer services such as reproductive toxicity testing. It should be noted that we can also provide customized gene therapy formulation toxicology services upon request.
Our Process of Gene Therapy Formulation Toxicology Services
Research design
- Objective determination
- Animal model selection
- Experimental protocol design
Dose and dosing regimen determination
Experimental implementation
- General toxicity tests
- Immunogenicity and immunotoxicity studies
- Genotoxicity tests
- Biodistribution and shedding studies
Data analysis and evaluation
- Toxicity response assessment
- Safety evaluation
Reporting and submission
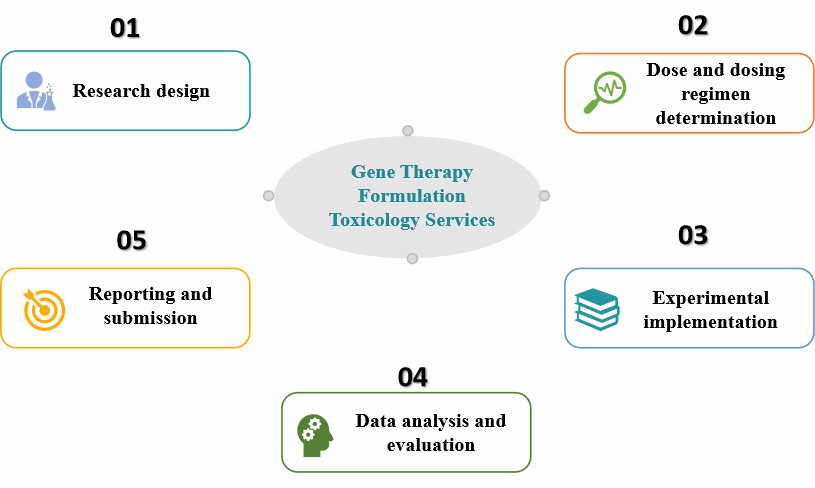 Fig.2 Our process of gene therapy formulation toxicology services. (CD Formulation)
Fig.2 Our process of gene therapy formulation toxicology services. (CD Formulation)
Our Platforms for Gene Therapy Formulation Toxicology Services
| Technologies & Platforms |
Content Description |
| Animal model construction |
We offer a wide range of animal models for in vivo efficacy and safety assessment of gene therapy products. |
| Pathology and toxicology platform |
We provide anatomic pathology, molecular pathology, and diagnostic pathology to support comprehensive toxicology evaluations. |
| Pharmacokinetics and bioanalytics |
We support drug metabolism and biodistribution studies through ELISA, electrochemiluminescence and other technologies. |
Highlights of Our Gene Therapy Formulation Toxicology Services
- Advanced technology platforms. We developed viral vectors to evaluate vector biodistribution, immune response, liver or neurotoxicity, etc. As well as by developing non-viral vectors to analyze vector stability, cellular uptake efficiency, and local stimulation response.
- Customized research protocols. Based on the mechanism of action and indication characteristics of the drug candidates, we offer dose exploration and toxicity marker screening, long-term safety tracking, and immunotoxicity-specific studies.
- Global compliance experience. We have completed toxicology studies for a number of gene therapy programs to date and are familiar with the review logic of the relevant regulatory authorities.
- An interdisciplinary team of experts. Our expert technical team includes toxicologists, gene therapy developers, and regulatory affairs consultants, providing collaboration from protocol design to report interpretation.
CD Formulation is committed to providing high-quality and efficient toxicology study services to our clients, and to facilitate the safe development of gene therapy formulations. If you are interested in us, please feel free to contact us.
Related Services


 Fig.2 Our process of gene therapy formulation toxicology services. (CD Formulation)
Fig.2 Our process of gene therapy formulation toxicology services. (CD Formulation) 