Gene Therapy Formulation Pharmacology Service
Inquiry
Preclinical pharmacological investigations play an integral role in the construction of gene therapy formulations, and their importance extends throughout the entire research and development process, profoundly affecting the success, harmlessness and operational feasibility of subsequent trials. Such investigations involve a series of sophisticated and complex assays aimed at revealing the mechanism of action, the mode of distribution in the organism, the safety profile, and the possible risks of toxicity of the gene therapy formulation.
CD Formulations, as an outstanding technical consultant and service provider in the field of cutting-edge biotechnology, is committed to providing strong support for the pharmacological evaluation and experimentation of gene therapeutic agents based on its deep expertise and advanced technology platform.
The Importance of Gene Therapy Formulation Pharmacology Service
Pharmacological analysis and testing of gene therapy formulations is not only a validation part of the quality and function of gene therapy products but also an important means to assess their safety and efficacy. The pharmacological properties of gene therapy formulations are decisive for their therapeutic efficacy in the host and have a profound impact on the design and prediction of subsequent experiments. We have explored the pharmacology of gene therapy formulations to comprehensively analyze their biological activity, mechanism of operation, in vivo distribution characteristics, and potential toxicity responses to ensure the safety and efficacy of gene therapy formulations.
Through pharmacological analysis, we can help researchers focus on their direct efficacy, but also dig deeper into how these compounds interact with host cells and how they are absorbed by different tissues and eventually metabolized, revealing their unique workings, and effectively avoiding possible risks, thus promoting this cutting-edge field of science and technology to a more mature and stable direction.
Explore Our Gene Therapy Formulation Pharmacology Service
Mechanism of action studies
Pharmacological evaluation of gene therapy formulations can help researchers gain insights into how these biologics interact with cells or tissues and achieve the desired efficacy by probing their inner workings. We are committed to providing our partners with detailed guidance on the pharmacological testing of gene therapy formulations covering, but not limited to, multiple dimensions of gene transcription, regulation of signaling pathways, and target affinity studies. A thorough understanding of the mechanism of action of gene therapy formulations not only helps to identify potential new therapeutic targets but also facilitates the optimization of formulation design, thus driving the entire gene therapy field towards greater precision and efficiency.
Biodistribution studies
Biodistribution studies are designed to evaluate the delivery efficiency of a gene vector or formulation in a target tissue by monitoring its localization and concentration changes in different tissues in vivo. The biodistribution of a gene therapy formulation determines its efficacy and safety. We quantify the presence of gene therapy formulations in vivo by using techniques such as radiolabeling, fluorescent labeling, or PCR quantification.
Safety and toxicology assessment
Safety and toxicology assessment is an important step in ensuring that gene therapy formulations are not harmful to patients. Tests include acute toxicity, chronic toxicity, and immunogenic response. Toxicology assessment analyzes in-depth the toxic side effects of the formulation under high dose or long-term administration through a series of animal and cellular experiments, to ensure the safety of gene therapy formulations in subsequent applications.
Immune response testing
Gene therapy formulations in the host may result in a response from the host immune system, so it is essential to assess immunogenicity at the preclinical stage. We commonly use in vitro immune cell response assays, ELISA, and flow cytometry for immunogenicity testing. We help investigators understand in advance that gene therapy formulations may elicit excessive immune responses so that formulation optimization can be adjusted in subsequent formulation development
 Fig.1 Our service of gene therapy formulation pharmacology analysis. (CD Formulation)
Fig.1 Our service of gene therapy formulation pharmacology analysis. (CD Formulation)
Our Technologies for Gene Therapy Formulation Pharmacology Service
| Platforms & Technologies |
Content Description |
| Molecular biology platforms |
We utilize advanced technologies such as PCR, quantitative polymerase chain reaction, and genome editing to accurately detect and analyze transgene expression, nucleic acid stability, and target binding to help customers perform highly sensitive gene expression and distribution analysis. |
| High-resolution imaging platform |
We can effectively assess the localization and distribution of gene therapy formulations in vivo with the help of fluorescence microscopy, in vivo imaging systems and other technologies. Our imaging technology platform enables real-time tracking of gene vectors in vivo without affecting the in vivo environment. |
| Cell biology and immunology platforms |
We analyze cytotoxicity, immunogenicity and cytokine release using flow cytometry, ELISA and other techniques. This technology platform of ours provides a reliable means of assessing the immune safety of gene therapy formulations. |
| Animal model platform |
We have established a variety of animal models that enable comprehensive testing of the biodistribution, safety and dose-effect relationships of gene therapy formulations. We can help our clients obtain more in vivo data through animal model experiments. |
Highlights of Gene Therapy Formulation Pharmacology Service
- We have extensive and specialized experience in drug discovery and development of gene therapy formulations.
- We have various disease evaluation models and animal models to support pharmacological efficacy and drug safety evaluation studies.
- We have various technology platforms for the integrated evaluation of gene therapy formulations and can develop personalized integrated evaluation research strategies for the characteristics of different types of gene therapy formulations.
- We follow the cutting-edge direction of gene therapy formulation development and have established advanced data and data acquisition management systems.
Published Data
Technology: Optimized pharmacological control
Journal: Mol Ther Methods Clin Dev
IF: 4.7
Published: 2021
Gene therapy is an irreversible process, and it is not possible to stop the treatment if adverse side effects occur or to adjust the expression level of the therapeutic agent to the needs of the individual patient. Therefore, the establishment of gene switch (GS) systems for pharmacologically modulating neurotrophic factor expression for the treatment of patients with Parkinson's disease is of great importance. Mifepristone, a synthetic steroid used to control transgene expression of GS carriers, is an approved clinical drug. However, the pharmacokinetics and kinetics of mifepristone vary widely among experimental animal species and depend on age and sex. In humans, mifepristone binds to high-affinity plasma carrier proteins, but not in any other species. In this article, researchers demonstrate through a series of experiments that the formulation of mifepristone can have a significant impact on its ability to activate the GS system. In addition, the authors show that the pharmacologic enhancer ritonavir (Rtv) can effectively potentiate the pharmacologic effects of mifepristone and allow it to overcome gender- and species-specific pharmacokinetic and kinetic problems.
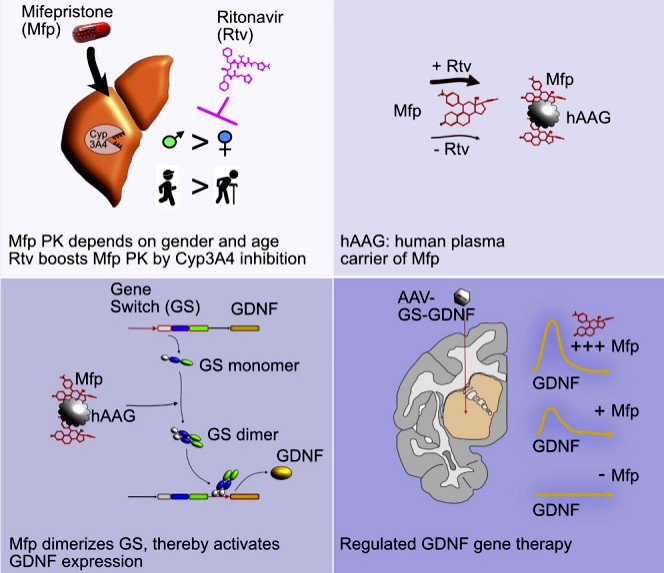 Fig.2 Regulable gene therapy may enable personalized genetic medicines. (Cheng S, et al., 2021)
Fig.2 Regulable gene therapy may enable personalized genetic medicines. (Cheng S, et al., 2021)
CD Formulation is committed to providing professional and reliable gene therapy formulation pharmacology services to support the development and innovation of gene therapy formulations. We provide solid technical support and solutions for the development of gene therapy formulations based on our philosophy of innovation, quality and efficiency. If you are interested in us, please feel free to contact us.
Reference
- Cheng S, et al. Optimized pharmacological control over the AAV-Gene-Switch vector for regulable gene therapy. Mol Ther Methods Clin Dev. 2021, 23:1-10.
Related Services

 Fig.1 Our service of gene therapy formulation pharmacology analysis. (CD Formulation)
Fig.1 Our service of gene therapy formulation pharmacology analysis. (CD Formulation)  Fig.2 Regulable gene therapy may enable personalized genetic medicines. (Cheng S, et al., 2021)
Fig.2 Regulable gene therapy may enable personalized genetic medicines. (Cheng S, et al., 2021) 