Gene Therapy Formulation Pharmacodynamic Assay
Inquiry
Gene therapy formulation pharmacodynamics assay can determine the effect of gene therapy formulation on the target disease, whether it achieves the expected effect, and also can design and optimize the gene therapy formulation through pharmacodynamics study. Pharmacodynamic assay of gene therapy formulations plays an essential role in meeting the regulatory requirements of relevant organizations and ensuring the efficacy of the products.
CD Formulation is committed to providing customized preclinical testing services for gene therapy formulation development. We have strong expertise in pharmacodynamic testing of gene therapy formulations and have accumulated rich research experience, which can provide high-quality data for your gene therapy formulation development and valuable guidance for subsequent study design.
The Importance of Gene Therapy Formulation Pharmacodynamic Assay
The pharmacodynamic assay provides a scientific basis for the efficacy and mechanism of gene therapy formulations by directly observing their biological responses in vitro or in vivo models. The importance of pharmacodynamic testing is reflected in the following aspects.
- Validation of the effectiveness of gene formulations. The biological activity and efficacy of gene therapy formulations can be effectively assessed through pharmacodynamic testing.
- Determining the minimum effective dose. Pharmacodynamic testing can help developers of gene therapy formulations determine the minimum effective dose to avoid toxic reactions caused by overdosing.
- Assess off-target effects and immunogenicity. Pharmacodynamic assays for gene therapy formulations are designed to achieve the desired effect and have a favorable safety profile, and pharmacodynamic testing to assess off-target effects and the risk of inducing an immune response can help investigators develop gene therapy formulations that meet the relevant requirements.
- Predict efficacy and durability. Pharmacodynamic tests predict the duration of action and persistence of efficacy of gene therapy formulations and are used to assess their stability and durability in organisms.
Explore Our Gene Therapy Formulation Pharmacodynamic Assay
Biomarker detection
By monitoring the expression levels of biomarkers, we can assess the efficacy of gene therapy formulations. Different biomarkers are required for different therapeutic targets, such as inflammation inhibition and tumor growth inhibition. We utilize multiple biomarker analysis techniques to ensure highly accurate data acquisition and interpretation.
Gene expression and protein activity assessment
Pharmacodynamic testing also requires an assessment of gene expression levels and therapeutic protein activity to determine the effectiveness of a gene-based formulation in target cells. For example, gene expression in transfected cells is assayed by qPCR, or the presence and activity of specific proteins is assayed by flow cytometry.
Immunogenicity testing
The immune response induced by a genetic formulation may result in adverse reactions or failure. Therefore, immunogenicity testing is central to pharmacodynamic testing of gene therapy. We use highly sensitive immunoassay platforms to help our clients effectively assess potential immune response risks.
Off-target effects and safety analysis
Off-target effects in gene therapy may lead to unintended genetic changes, thus posing unpredictable safety risks. To guarantee a gene formulation's target specificity, a number of molecular assays can be used to track how it affects areas other than the target cells. Off-target risks are frequently tightly controlled with multiplexed tests.
In vivo efficacy assays
In addition to in vitro assays, in vivo pharmacodynamic testing is also essential. The distribution, efficacy and safety of the drug are assessed by injecting the gene formulation into an animal model and observing its effect on the target tissue.
 Fig.1 Our service of gene therapy formulation pharmacodynamic assay. (CD Formulation)
Fig.1 Our service of gene therapy formulation pharmacodynamic assay. (CD Formulation)
Our Technologies for Gene Therapy Formulation Pharmacodynamic Assay
| Platforms & Technologies |
Content Description |
| Flow cytometry |
We utilize flow cytometry to allow for the simultaneous detection of multiple cytokines, assessment of immune cell activity, gene expression levels, etc., which helps to evaluate the immunogenicity and activity of genetic formulations. |
| High-intensity imaging |
We utilize high-content imaging technology, which combines imaging and analytical functions for observing gene expression or protein distribution at the cellular level and is suitable for multifactorial impact analysis in pharmacodynamic experiments. |
| Animal model research platform |
We have an animal experiment platform that meets international standards and is suitable for all kinds of animal model testing, such as mice and rabbits. In vivo experiments provide data support on the long-term effects of gene therapy agents on organisms, pharmacokinetics and so on. |
Highlights of Gene Therapy Formulation Pharmacodynamic Assay
- We have established a comprehensive R&D platform for gene therapy formulations, providing one-stop services such as pharmacological efficacy, biodistribution and safety evaluation studies.
- We have abundant animal models and a variety of advanced analytical technologies, which enable us to complete multiple preclinical testing projects for gene therapy formulation development, taking into account the characteristics of different R&D projects.
- We have nucleic acid level, cell level, protein level analysis platforms and advanced instrumentation to ensure the accuracy of experimental results.
- We provide bioanalytical services in compliance with international quality management standards, including biomarker studies and preclinical pharmacology and efficacy studies.
Published Data
Technology: Pharmacodynamic assay
Journal: J Antimicrob Chemother
IF: 5.2
Published: 2016
One promising approach to curing HIV is the use of engineered nucleic acid endonucleases that bind to and cleave specific target sequences within the latent genome, resulting in mutations that prevent the virus from replicating. In this article, the authors developed a mechanistic model that can predict the number of copies of a transgene expressed at a given dose in a single target cell based on the fluorescence of the reporter gene. The authors fit the model to flow cytometry datasets to determine the optimal vector serotype, promoter, and dose required to achieve maximum expression. The results show that the model provides a more accurate measure of transduction efficiency, enables the selection and optimization of quantitative doses, and can be easily applied to various other gene therapy applications.
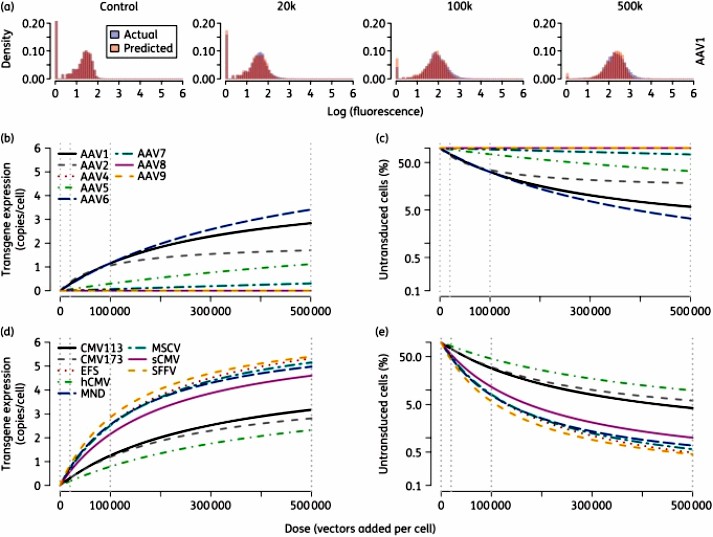 Fig.2 Delivery and expression with various adeno-associated virus serotypes and promoters. (Roychoudhury P, et al., 2016)
Fig.2 Delivery and expression with various adeno-associated virus serotypes and promoters. (Roychoudhury P, et al., 2016)
CD Formulation is dedicated to providing reliable technical support and comprehensive solutions for pharmacodynamic analysis of gene therapy formulations. If you are interested in us, please feel free to contact us.
Reference
- Roychoudhury P, et al. Pharmacodynamics of anti-HIV gene therapy using viral vectors and targeted endonucleases. J Antimicrob Chemother. 2016, 71(8):2089-99.
Related Services

 Fig.1 Our service of gene therapy formulation pharmacodynamic assay. (CD Formulation)
Fig.1 Our service of gene therapy formulation pharmacodynamic assay. (CD Formulation)  Fig.2 Delivery and expression with various adeno-associated virus serotypes and promoters. (Roychoudhury P, et al., 2016)
Fig.2 Delivery and expression with various adeno-associated virus serotypes and promoters. (Roychoudhury P, et al., 2016)