Liposomes and Lipid Nanoparticles for Therapeutic Research
Inquiry
CD Formulation specializes in liposome and lipid nanoparticle development and harbors extensive experience in the technologies for synthesizing liposomes and lipid nanoparticles. We leverage the leading lipid nanoparticle technologies to fabricate liposomes and lipid nanoparticles to accelerate therapeutic research.
Applications of Liposomes and Lipid Nanoparticles
Lipid nanoparticles effectively deliver different drugs to the targeted areas. They are widely applied in various disease therapies, including cancer therapy, bone therapy, fungal treatment, eye therapy, pain therapy, etc.
Cancer Therapy
Lipid nanoparticles have been suitable carriers of deliver DNA, mRNA, and siRNA for the treatment of various types of cancer, including gastric and esophageal cancer, pancreatic cancer, liver cancer, nervous system cancer, lung cancer, breast cancer, prostate cancer, etc.
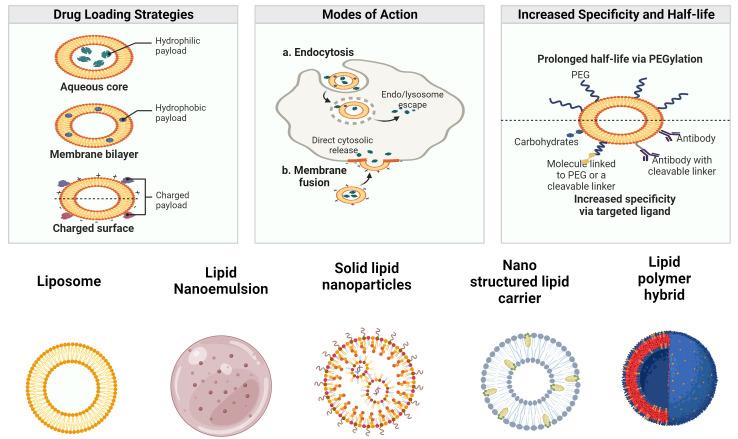 Fig.1 Drug delivery of LBNPs and their types. (Ibtesam Waheed, et al. 2024)
Fig.1 Drug delivery of LBNPs and their types. (Ibtesam Waheed, et al. 2024)
Bone Diseases Treatment
Lipid nanoparticles effectively deliver the drugs to the specific area to bone disease treatment, such as osteoarthritis, osteoporosis, and bone cancer.
Fungal Treatment
Lipid nanoparticles can improve permeability and overcome various challenges in skin drug delivery.
Eye Therapy
Lipid nanoparticles are suitable for ophthalmic drug delivery to treat various eye diseases.
Pain Therapy
We have investigated targeting drug-loaded lipid nanoparticles to specific sites in the body to improve toxicity or side effects, increase drug uptake or bioavailability, and provide prolonged drug release.
Marketed Liposome and Lipid Nanoparticle Products for Disease Treatment
Many liposome and lipid nanoparticle products for the different disease treatment have been successfully developed and launched.
Table 1. Marketed liposome and lipid nanoparticle products. (Yuanchao Jia, et al. 2023)
| Trade Name |
Approval Year |
Drug Agent |
Company |
Clinical Applications |
Agency |
Administration Route |
| Ambisome@ |
1990 |
Amphotericin B |
Gilead Sciences(Foster CA,USA) |
Fungal infection/Anti-leishmanial |
EMA |
intravenous |
| Epaxal@ |
1993 |
Inactivated hepatitis A virus (strain RGSB) |
Crucell Berna Biotech(Berne, Switzerland) |
Hepatitis A |
EMA |
intramuscular |
| Abelcet |
1995 |
Amphotericin B |
Sigma-Tau Pharmaceutical Inc. (Gaithersburg,MD,USA) |
Invasive severe fungal infections |
FDA |
intravenous |
| Doxil /Caelyx |
1995/1996 |
Doxorubicin |
Sequus Pharmaceuticals (Santa Clara County,CA,USA) |
Ovarian cancer and KS |
FDA/EMA |
intravenous |
| Amphotec@ |
1996 |
Amphotericin B |
Ben Venue Laboratories (Bedford,OH,USA) |
Severe fungal infections |
FDA |
intravenous |
| DaunoXome |
1996 |
Daunorubicin |
NeXstar Pharmaceuticals (Foster,CA,USA) |
KS infected with HIV |
FDA |
intravenous |
| Inflexal v |
1997 |
Inactivated hemagglutinin of Influenza virus strains A and B |
Crucell Berna Biotech (Berne, Switzerland) |
Influenza |
EMA |
intramuscular |
| Depocyt |
1999 |
Cytarabine |
Skye Pharm Inc. (San Diego CA,USA) |
Neoplastic meningitis |
FDA |
spinal |
| Visudyne" |
2000 |
Verteporfin |
Novartis AG (Basel, Switzerland) |
Choroidal neovascularization |
FDA |
intravenous |
| Myocet |
2001 |
Doxorubicin |
IDM Pharma(Irvine CA,USA) |
Combination therapy with cyclophosphamide in metastatic breast cancer |
EMA |
intravenous |
| Lipusu |
2003 |
Paclitaxel |
Luye Pharma(Nanjing,China) |
Ovarian cancer |
NMPA |
intravenously guttae |
| DepoDurIM |
2004 |
Morphine Sulfate |
SkyPharm Inc. (San Diego, CA,USA) |
Pain management |
FDA |
Epidural |
| Mepact |
2009 |
Mifamurtide |
Elan Pharmaceuticals (San Diego,CA,USA) |
Non-metastatic osteosarcoma |
EMA |
intravenous |
| Exparel |
2011 |
Bupivacaine |
Pacira BioSciences (San Diego CA,USA) |
Pain management |
FDA |
intravenous |
| Marqibo |
2012 |
Vincristine |
Talon Therapeutics (San Francisco,CA,USA) |
ALL |
FDA |
intravenous |
| OnivydeIM |
2015 |
Irinotecan |
Merrimack Pharmaceuticals (Cambridge,UK) |
Metastatic pancreatic cancer |
FDA |
intravenous |
| Vyxeos⑩ |
2017 |
Daunorubicin and Cytarabine |
Jazz Pharmaceuticals (San Francisco,CA,USA) |
AML-MRC and t-AML |
FDA |
intravenous |
| Shingrix |
2017 |
Recombinant VZV glycoprotein E |
Glaxo Smith Kline (Middlesex, UK) |
Against shingles and post-herpetic neuralgia |
FDA |
intramuscular |
| OnpattroTM |
2018 |
siRNA |
Alnylam(Cambridge, MA,USA) |
Polyneuropathy caused by hATTR |
FDA |
intravenous |
| Arikayce@Kit |
2018 |
Amikacin |
Insmed (Glen Allen,VA,USA) |
NTM lung disease caused by MAC |
FDA |
Inhalation administration |
| Mosquirix |
2021 |
Recombinant CSP |
Glaxo Smith Kline(Middlesex, UK) |
Malaria |
EMA |
intramuscular |
| Comirnaty@ |
2021 |
BNT162b2 |
Pfizer(New York,NY,USA) and BioNTech(Mainz, Germany) |
COVID-19 |
FDA |
intramuscular |
| mRNA-1273 |
2021 |
mRNA-1273 |
Moderna(Cambridge, MA,USA) |
COVID-19 |
FDA |
intramuscular |
FDA: Food and Drug Administration; EMA: European Medicines Agency; NMPA: National Medical Products Administration
Custom Lipid Nanoparticle Development Service for Disease Treatment
Lipid nanoparticles and liposomes are widely supplied in the treatment of various diseases, such as cancer therapy, eye therapy, pain therapy, etc. At our mature liposome and lipid nanoparticle technology platform, CD Formulation has been committed to developing liposomes and lipid nanoparticles for various disease therapies, aiding customers' different personalized needs and promoting the rapid development and applications of liposomes and lipid nanoparticles.
Liposome Development for Nanomedicine
At CD Formulation's platform, our team is keen on the research and development of liposomes for the treatment of various diseases. After our continuous experimental accumulation and gradual improvement of the liposome development process, we can customize and develop personalized liposomes for different customers.
Lipid Nanoparticle Development for Nanomedicine
At this technology platform, CD Formulation has explored and researched the fabrication techniques of lipid nanoparticles for treating different diseases. And we can offer personalized lipid nanoparticle customization services for different customers.
Highlights for Liposomes and Lipid Nanoparticles in Disease Treatment
- By our advanced liposome and lipid nanoparticle technologies, we provide our high-quality liposome and lipid nanoparticle customerization services for theraceutic research.
- We also have rich experience in exploring and researching the verity of the fabrication technologies of functional liposomes and lipid nanoparticles according to the characterization of different disease treatments, such as cancer therapy, bone therapy, eye therapy, pain therapy, etc.
Published Data
Technology: Fabrication of lipid nanoparticles and liposomes for bone diseases treatment
Journal: Biomedicines
IF: 3.9
Published: 2022
Results:
The authors discuss the applications of lipid nanoparticle and liposome systems based on different active substances in bone diseases. Lipid nanoparticles have biocompatibility, sufficient drug release control, and passive targeting capabilities, making them one of the most widely used drug delivery systems in the world, especially in promoting tissue regeneration and treating diseases.
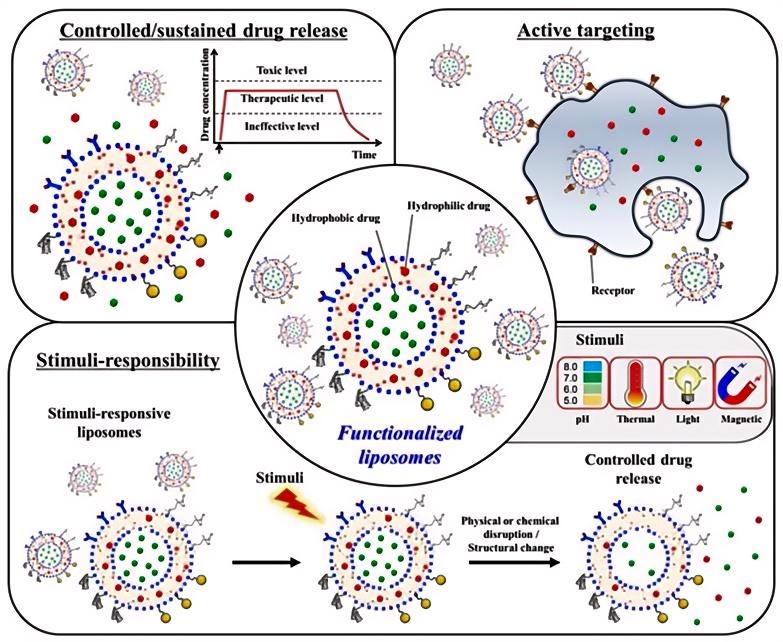 Fig.2 Scheme of functionalized liposomes used in bone regeneration applications. (Alexandra-Cristina Burdușel, et al. 2022)
Fig.2 Scheme of functionalized liposomes used in bone regeneration applications. (Alexandra-Cristina Burdușel, et al. 2022)
Liposomes and lipid nanoparticles are widespread used as delivery carriers for a variety of drugs due to their unique advantages. Based on vast experience and solid technical strength, CD Formulation can offer liposome and lipid nanoparticle formulation development services for treating various diseases. If you are interested in liposomes and lipid nanoparticles for therapeutic research, please contact us.
References
- Ibtesam Waheed, Anwar Ali, Huma Tabassum, et al. Lipid-based nanoparticles as drug delivery carriers for cancer therapy. Front Oncol. 2024,14: 1296091.
- Yuanchao Jia, Yuxin Jiang, Yonglong He, et al. Approved Nanomedicine against Diseases. Pharmaceutics. 2023,15,774.
- Alexandra-Cristina Burdușel, Ecaterina Andronescu. Lipid Nanoparticles and Liposomes for Bone Diseases Treatment. Biomedicines. 2022,10(12): 3158.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Drug delivery of LBNPs and their types. (Ibtesam Waheed, et al. 2024)
Fig.1 Drug delivery of LBNPs and their types. (Ibtesam Waheed, et al. 2024)  Fig.2 Scheme of functionalized liposomes used in bone regeneration applications. (Alexandra-Cristina Burdușel, et al. 2022)
Fig.2 Scheme of functionalized liposomes used in bone regeneration applications. (Alexandra-Cristina Burdușel, et al. 2022)
