Liposome Development for Nanomedicine
Inquiry
CD Formulation can provide our clients with liposome development services for nanomedicines to meet the different requirements using our advanced liposome fabrication technology. Our highly qualified nanoformulation development team can design and prepare liposomes based on different loads, thus promoting research and even commercialization of liposomes for nanomedicine.
Advantages of Liposomes as Drug Carriers
Liposomes for nanomedicine are composed of a unilamellar or bilamellar phospholipid bilayer and an aqueous core. Liposomes have properties such as low toxicity, high biocompatibility, and the ability to carry both lipophilic and hydrophilic compounds, making them potential carriers for many drugs, such as therapeutic proteins, diagnostics, anticancer drugs, and other drugs.
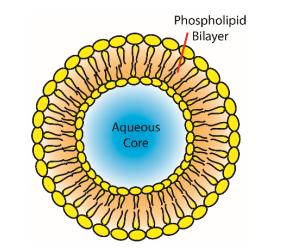 Fig.1 Structure of a liposome. (Ada W.Y. Leung, et al, 2019)
Fig.1 Structure of a liposome. (Ada W.Y. Leung, et al, 2019)
- Liposomes can provide selective passive targeting to tumor tissues.
- Liposomes can increase efficacy, therapeutic index and stability via encapsulation.
- Liposomes can reduce in toxicity of the encapsulated agents.
- Liposomes have the ability to carry both lipophilic and hydrophilic compounds.
- Liposomes can improve pharmacokinetic effects (reduced elimination, increased circulation lifetimes).
- Liposomes can enhance flexibility to couple with site specific ligands to achieve active targeting.
- Liposomes have high biocompatibility, biodegradability, and decreased drug side effects.
Our Workflow for Liposomal Drug Product Development
As a leading pharmaceutical laboratory services company, we provide you with comprehensive, innovative, and timely solutions and help you quickly customize high-quality liposomes for nanomedicine. We support all phases of the formulation development and even the commercialization process of liposomal nanomedicines through our extensive technical expertise in liposome technology. There are the main steps for liposome development for nanomedicine, including synthesis, formulation, synthesis and sterilization, storage, resuspension, and administration.
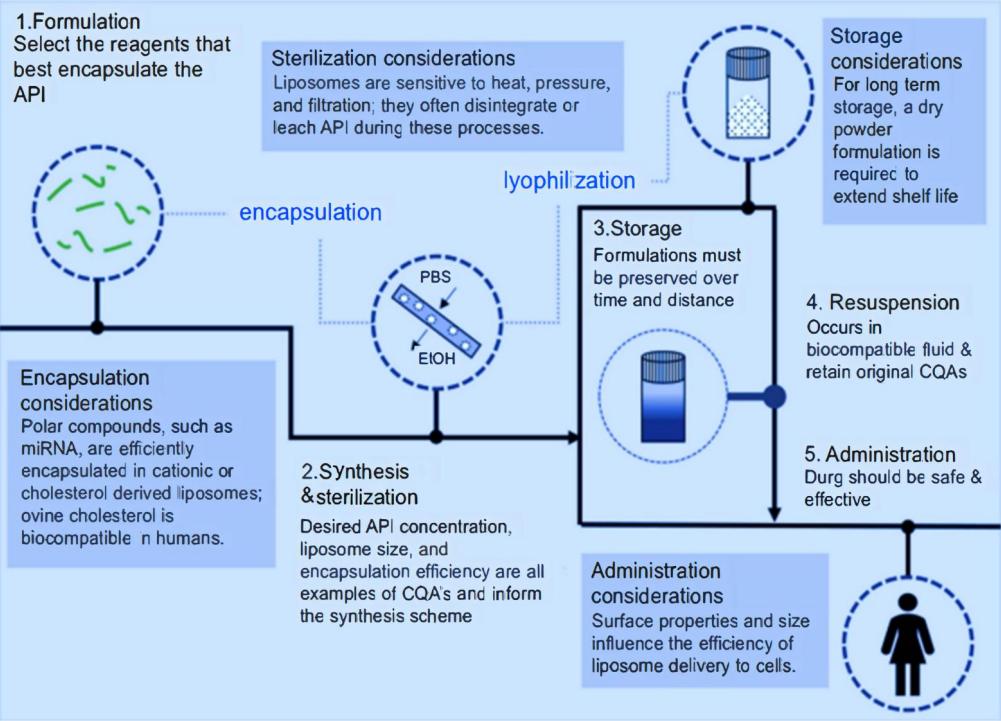 Fig.2 The flowchart for liposomal drug product development. (Henry Lujan, et al. 2019)
Fig.2 The flowchart for liposomal drug product development. (Henry Lujan, et al. 2019)
Our Methods for Liposome Preparation
There are various methods for preparing liposomes, including thin-film hydration method, reverse-phase evaporation, ethanol injection, supercritical fluid-based methods, microfluidic method, etc. CD Formulation relies on our advanced design concept to use the following preparation methods according to different nanomedicine properties to prepare liposomes that meet your requirements.
Conventional Methods of Liposome Preparation
Thin film hydration method: In this method, lipids, including phospholipids, are dissolved and mixed in an organic solvent such as chloroform. The solvent is then evaporated to form a lipid gel. The gel is hydrated with an aqueous medium containing functional substances and agitated to regenerate the lipids and form liposomes.
Reverse phase evaporation: In reversed phase evaporation, functional substances are encapsulated by forming reverse micelles in the organic phase. Liposomes are formed by mixing an aqueous core medium with an organic solvent containing phospholipids, evaporating the organic solvent and suspending the lipids in an aqueous dispersion medium, followed by extrusion.
Ethanol injection: In this method, phospholipids, other lipids, and functional substances are dissolved in ethanol and injected into a stirred aqueous dispersion medium using a syringe or equivalent device, forming liposomes by gradually reducing the ethanol concentration.
Supercritical Fluid-Based Methods of Liposome Preparation
Supercritical fluids exhibit characteristics above their critical temperature and pressure of gases and liquids, and they can penetrate porous solids such as gases and dissolve other substances such as liquids. The preparation method of liposomes based on supercritical fluids utilizes the different solubilities of lipids and organic solvents in supercritical fluids to prepare liposomes.
Supercritical antisolvent method: This method uses supercritical fluids as antisolvents to produce precursor liposomes by reducing the solubility of lipids dissolved in organic solvents. Liposomes are then prepared by hydrating the precursor liposomes in an aqueous dispersion medium.
Supercritical reverse-phase evaporation: This method uses supercritical carbon dioxide as a co-solvent to mix lipids dissolved in organic solvents with supercritical carbon dioxide in a high-pressure stirring vessel. The aqueous core medium containing functional substances is sprayed into the container. The container is then depressurized, resulting in the formation of liposomes in the aqueous dispersion medium contained in the container.
Microfluidic Method of Liposome Preparation
This method is to continuously produce liposomes and precisely control the size of liposomes by exploiting the unique phenomenon of the interface between liquids. We can prepare liposomes by flowing an organic solvent containing phospholipids through microchannels together with an aqueous phase.
In addition to the above methods for preparing liposomes, we are committed to researching emerging technologies to explore green synthesis technologies for liposomes to provide emerging professional support for researchers.
Our Analytical Capabilities for Liposome Formulations
The physical stability of liposomes during prolonged storage can be determined by monitoring changes in particle size, zeta potential and appearance as a function of time. CD Formulation can provide you with the following analysis and characterization of liposomes including:
- Particle Size
- Zeta Potential
- Encapsulation Efficiency
- Drug Loading
- In Vitro Drug Release
- Stability
Why Choose Us to Develop Liposomes?
- Relying on our strong drug innovation technology and integrating superior resources, CD Formulation has built a comprehensive liposome nanoformulation research and development platform with advanced technology, complete supporting facilities, complete functions, interconnection and standardized operation. We can provide liposome research and development, liposome new dosage form preparation research and development, liposome generic drug research and development and other services.
- Our team of elite experts utilizes advanced equipment to quickly design and construct methods for liposome preparation, such as thin-film hydration, reverse-phase evaporation, ethanol injection, supercritical fluid-based methods, etc.
- We offer analysis and characterization services for liposomes to ensure our customized liposomes meet your requirements.
- All experiments are signed confidentiality agreements, focusing on protecting customer privacy.
Published Data
Technology: Recent liposome production approaches, such as, Liposomes on chips, Flow focusing, Pulsed jetting, etc.
Journal: Advanced Drug Delivery Reviews
IF: 16.1
Published: 2020
Results:
The authors review the latest liposome preparation technologies, excipients for liposome formulations used in various new studies, and administration routes used to deliver liposomes to target disease areas. The authors also describe the optimization of liposome-based drug delivery systems, preparation techniques with higher reproducibility, and broader new modal applications including nucleic acid therapy, CRISPR/Cas9 therapy, and immunotherapy.
CD Formulation is willing to be your most loyal partner in liposomal formulation development. If you have a requirement about our services, please contact us by phone or email, we are looking forward to in-depth communication and cooperation with you.
References
- Ada W.Y. Leung, Carolyn Amador, Lin Chuan Wang, et al. What Drives Innovation: The Canadian Touch on Liposomal Therapeutics. Pharmaceutics. 2019,11,124.
- Henry Lujan, Wezley C Griffin, Joseph H Taube, et al. Synthesis and characterization of nanometer-sized liposomes for encapsulation and micro RNA transfer to breast cancer cells. International Journal of Nanomedicine. 2019, 14: 5159-5173.
- Nina Filipczak, Jiayi Pan, Satya Siva Kishan Yalamarty, et al. Recent advancements in liposome technology. Advanced Drug Delivery Reviews. 2020, 156: 4-22.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Structure of a liposome. (Ada W.Y. Leung, et al, 2019)
Fig.1 Structure of a liposome. (Ada W.Y. Leung, et al, 2019) Fig.2 The flowchart for liposomal drug product development. (Henry Lujan, et al. 2019)
Fig.2 The flowchart for liposomal drug product development. (Henry Lujan, et al. 2019)
