Lyophilized Liposome Formulation Development
Inquiry

The freeze-drying development technology of CD Formulation helps customers reduce the stability risks of known liposomal formulations and improve the shelf life of liposomal drugs. Currently, our freeze-drying technology can be used for different routes of administration (i.e. parenteral, oral, nasal and/or pulmonary). With decades of drug development experience and comprehensive expertise, CD Formulation is committed to developing best-in-class freeze-dried liposomes to meet your pharmaceutical needs. Whether you want to improve drug bioavailability, reduce drug side effects, or improve drug stability, we can provide you with the best solution. Our goal is to provide our customers with high-quality and efficient freeze-dried formulations to meet different needs and application scenarios.
Why Develop Lyophilized Liposome Formulations?
Formulation development is the key step of drug research and development. At present, there are still some limitations in the development of liposomes that may alter the biological distribution of drugs in the body and thus their efficacy and safety, such as lipid oxidation or hydrolysis, drug leakage, aggregation or vesicle fusion, etc. When formulation development methods (i.e. use of saturated and/or high transition phase lipids, addition of antioxidants, design of charged and functionalized particles, etc.) or preventative storage conditions (storage in an inert atmosphere, -20°C, protection from light) are not sufficient to overcome the above problems, our only option is to drying liposomes (because all of the above phenomena are prone to occur in aqueous environments). In the formulation development of lyophilized liposomes, it is critical to select the appropriate excipients and optimal process parameters to protect the integrity of the liposome membrane and from stresses caused by freezing and dehydration.
Our Lyophilized Liposome Formulation Development Services
Based on our extensive experience in the development of pharmaceutical freeze-drying processes, you will benefit from the expertise and advanced platform of our excellent scientific team in developing your lyophilized liposome formulations. We will develop the optimal formula for lyophilized liposomes on the physicochemical properties of your active ingredient and provide you with multiple formulation variants according to routes of administration, in addition to optimizing the process parameters for the final delivery of sufficient long-term stable liposomes.
Freeze-dried liposome products retain all non-volatile formula ingredients in the formula. Therefore, the stability of the chemical composition is determined by formulation control.
During lyophilization, the formation of ice crystals, the change of osmotic pressure, phase separation and phase transition can lead to the folding, fusion, rupture and drug leakage of the liposome membrane. If suitable lyophilized protective agent is added before lyophilization and appropriate technology is adopted, the damage of lyophilized liposomes can be greatly reduced or even eliminated. After rehydration, the morphology, particle size and encapsulation rate of liposomes have no significant changes. We can be used to help you select suitable lyophilized protectants for your formulation of lyophilized liposomes.
For liposomes, the structural stability of the lipid carrier in the dry state, as well as the lipid bilayer recombination without leakage of the drug when water is added before and after administration, are the key features of lyophilization specific to the stability of this dosage form, so we will provide you with the characterization services of liposomes before and after lyophilization.
Our Workflow of Lyophilized Liposome Formulation Development
Liposome freeze-drying includes three processes, namely, pre-freezing, preliminary drying and secondary drying. Lyophilized liposomes can be used directly as solid dosage forms such as sprays or can be used after reconstituting liposome suspension with water or another suitable solvent hydration. However, the processes of pre-freezing, drying and rehydration are not conducive to the stability of the structure and function of liposomes, so reconstitution solutions are essential after freeze-drying process.
At CD Formulation, the formulation development of lyophilized liposomes is mainly divided into the following three steps: formulation design (including screening of various excipients, etc.), lyophilization process development (screening and optimization of lyophilization parameters, etc.), and reconstitution solutions (final evaluation of lyophilized products, etc.).
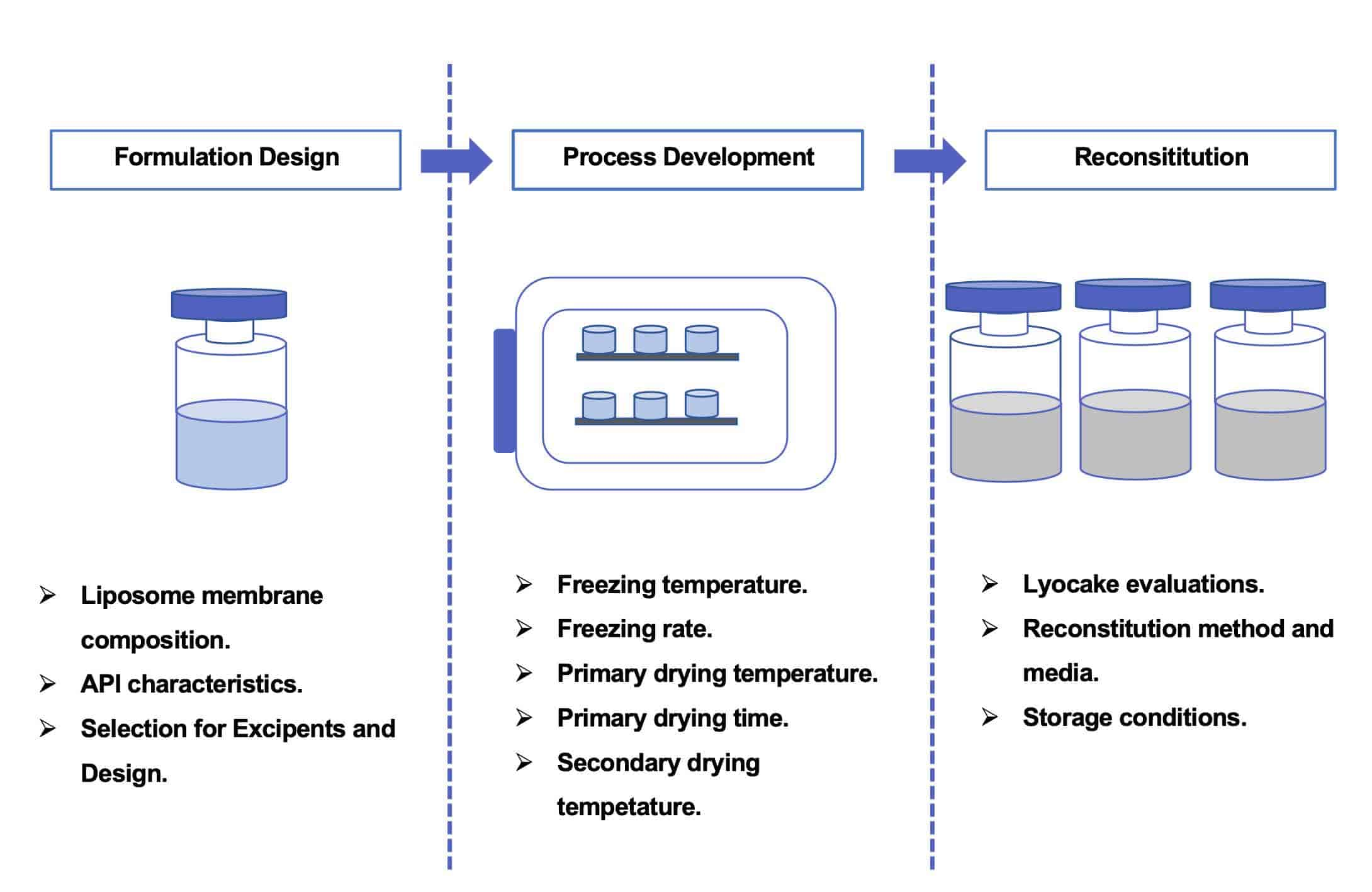 Fig.1 The workflow of lyophilized liposome formulation development. (CD Formulation)
Fig.1 The workflow of lyophilized liposome formulation development. (CD Formulation)
Our Lyophilization Technology Platforms
| Lyophilized Liposome Formulation Development Platform |
We provide you with both liposome formulation development and exclusive freeze-drying process development services for liposome to simultaneously meet your needs for liposomes membrane formulation design and excipient screening design. |
| Lyophilization Cycle Optimization Platform |
With a specialized freeze-drying development center, our scientists are able to develop freeze-drying cycles at laboratory, pilot, and production scales, while optimizing process parameters at the early development stage to scale up. We are committed to designing and developing robust and scalable freeze-drying cycles for smooth and efficient process transfer. |
| Lyophilized Liposome Reconstitution Platform |
Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a specific concentration of liquid, which is a vital step for lyophilized liposomes. We will provide your liposomes with freeze-dried reconstitution method design, as well as a variety of solvents for you to screen. |
| Lyophilized Liposome Analysis and Evaluation Platform |
Comprehensive analytical characterization of lyophilized liposomes is essential to distinguish between stable and unstable samples. We can offer multiple analysis methods to confirm the stability of lyophilized liposomes. Our analytical laboratory center is equipped with a variety of advanced equipment. |
Our Advantages in Lyophilized Liposome Formulation Development
- Advanced technology. Unique platform and professional equipment.
- Expertise. Proven research portfolio solutions and expert teams in lyophilized liposomes development.
- Flexibility. Collaborative and customized project design successful project execution.
Applications of Lyophilized Liposome Formulation
- Liposome drugs. Freeze-drying method improves the storage stability of liposomes. Making freeze-dried liposomes can significantly reduce the hydrolysis and oxidation rates of phospholipids and drugs.
- Diagnostic & Biological reagents. The main active components of diagnostic reagents (such as enzymes, antigens, antibodies) are dependent on low temperature environment, so it is particularly suitable for diagnostic reagents.
- Liposome cosmetics. Freeze-drying technology is beneficial for the preservation of cosmetic liposomes that require low temperature storage, such as peptides, enzymes, etc.
Published Data
Technology: Freeze-dried Technology
Journal: Pharmaceutics
IF: 5.4
Published: 2024
Results: In this study, the author encapsulated plant oils in lyophilized liposomes to enhance their stability and functional benefits, such as skin hydration and anti-aging effects, for innovative cosmetic formulations. The developed lyophilized liposomes underwent in vitro analysis, revealing that the gel-type formulations loaded with dried liposomes exhibited improved skin hydration, enhanced barrier function, and refined micro-texture, thereby suggesting an ameliorated skin condition.
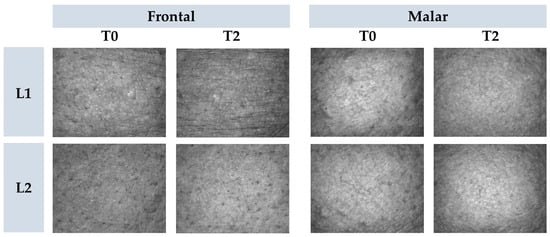 Fig.2 Representative images of cutaneous microrelief at the initial time (T0) and 2 h (T2) after application of vehicle gel formulation (L1) and gel containing dried liposomes with pequi oil (L2). (Kakuda, L., et al., 2024).
Fig.2 Representative images of cutaneous microrelief at the initial time (T0) and 2 h (T2) after application of vehicle gel formulation (L1) and gel containing dried liposomes with pequi oil (L2). (Kakuda, L., et al., 2024).
Our scientists at CD Formulation are happy to discuss your questions and inquiries related to lyophilized liposome formulation development services. If you have a requirement about our services, please contact us by phone or email, our colleagues will reply to you within three working days.
References
- Kakuda, L., Maia Campos, et al. Development and Efficacy Evaluation of Innovative Cosmetic Formulations with Caryocar brasiliense Fruit Pulp Oil Encapsulated in Freeze-Dried Liposomes. Pharmaceutics. 2024, 16, 595.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services






