Pre- and Post-freezing Liposome Characterization Service
Inquiry
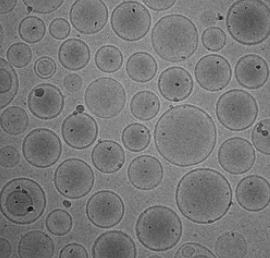
CD Formulation has accumulated extensive expertise in the development of liposome formulations. Currently, we possess the capability to encapsulate diverse drug molecules, including both small molecule drugs and macromolecular biological drugs, for various therapeutic categories. In our pursuit of ocular liposome development, we have consistently tackled challenges and successfully assisted numerous clients in resolving related issues. These include enhancing corneal adhesion and penetration of ocular liposomes, prolonging intravitreal half-life, as well as achieving targeted delivery through surface modification technology. Over the years, we have remained committed to innovation and leveraged cutting-edge technologies to explore the potential of next-generation liposome delivery systems in ocular formulations.
Why Pre- and Post-freezing Liposome Characterization Needed?
Liposomes, as a crucial drug carrier, have been extensively utilized in the field of drug delivery. The characterization services for liposomes play a vital role in the formulation development of lyophilized liposomes. Throughout the process of freeze-drying, liposomes undergo various physical and chemical changes that directly impact their stability, bioactivity, and drug efficacy. Pre-freezing liposome characterization services encompass assessing liposome morphology, size distribution, surface properties, and other aspects. These parameters reflect the physical properties and preparation process of liposomes and offer significant guidance for optimizing freeze-dried liposome formulations. Moreover, pre-freezing liposome characterization can evaluate drug loading capacity and release behavior to provide essential information for enhancing drug delivery effectiveness. Post-freezing liposome characterization services are equally important since drying, crystallization, vitrification processes during freeze-drying affect the stability, bioactivity, and drug efficacy of liposomes. Post-freezing characterization evaluates structural integrity, membrane fluidity as well as drug release behavior among other properties of freeze-dried liposomes. This information aids researchers in optimizing preparation processes and improving overall drug delivery effectiveness.
Our Services for Pre- and Post-freezing Liposome Characterization
List of available services for pre- and post-freezing liposome characterization:
| Service Categories |
Techniques |
Specific Services |
| Physical Characterization |
Liposome visualization techniques (transmission electron microscopy (TEM), environmental scanning electron microscopy (ESEM), confocal laser scanning microscopy (CLSM), atomic force microscopy (AFM), etc); phosphorus nuclear magnetic resonance technology; IR technology; static light scattering gel exclusion chromatography; light microscopy laser diffraction; small-angle X-ray; capillary electrophoresis, ICP-MS, etc. |
Liposome layer determination; viscosity; phase transition temperature of phospholipid; Zeta potential; particle size distribution and average particle size; osmotic pressure; liposome rheology; surface tension; morphological observation of liposome under an electron microscope; spectral data of the target liposome structure; solvent residue pre freeze-drying and moisture residue post freeze-drying lyocake morphology. |
| Chemical characterization |
HPLC analysis technology, HPLC-MS/MS analysis technology, GC analysis technology, GC-MS technology, MALDI-TOF MS technology, etc. |
Drug loading rate; Entrapment rate Impurity study; sterility/pyrogen/endotoxin; salt concentration changes in liposomes associated with lipid or API-related degradation products; Changes in the integrity of liposomes (e.g. drug release, drug loading rate, and liposome size). |
| In vitro Release Evaluation |
Sampling separation dialysis technology, Ussing chamber technology, Franz diffusion cell technology, flow cell technology, combination technology, and electrochemical technology. |
Evaluation of release in vitro and analysis of liposome permeability before and after lyophilization. |
| Stability Evaluation |
HPLC, HPLC analysis technology, HPLC-MS/MS analysis technology, MALDI-TOF MS technology, microbial culture technique, etc. |
The liposome leakage rate, lipid component stability, microbiological stability, physical attributes (such as liposome integrity, particle size distribution, fatty acid unsaturation), and chemical stability should be evaluated both before and after freeze-drying to ensure product quality throughout its validity period. |
Why Choose Us to Characterize Pre- and Post-freezing Liposome?
- Comprehensive Expertise. The experts possess extensive experience and teams are capable of conducting multidimensional evaluations.
- Cutting-edge Technology. The utilization of our cutting-edge analysis and evaluation platform enables us to effectively cater to the diverse analytical requirements of our esteemed clientele.
- Customized Solutions. The service is highly flexible and can be customized to meet your exclusive needs.
- Stringent Quality Control. Strict quality control measures throughout the development and manufacturing process.
- High efficiency. High efficiency project management and streamlined processes.
Published Data
Technology: rapid screening of liposomes using a scale down approach (microplates)
Journal: International Journal of Pharmaceutics
IF: 10.5
Published: 2020
Results: In this study, the author developed a high-throughput screening method using microplate technology to evaluate formulations for freeze-drying of empty liposomes and protein-lipid liposomes. This method involves the screening and determination of optimal formulation and process parameters for freeze-drying, including factors such as liposome charge and concentration before and after the drying process.
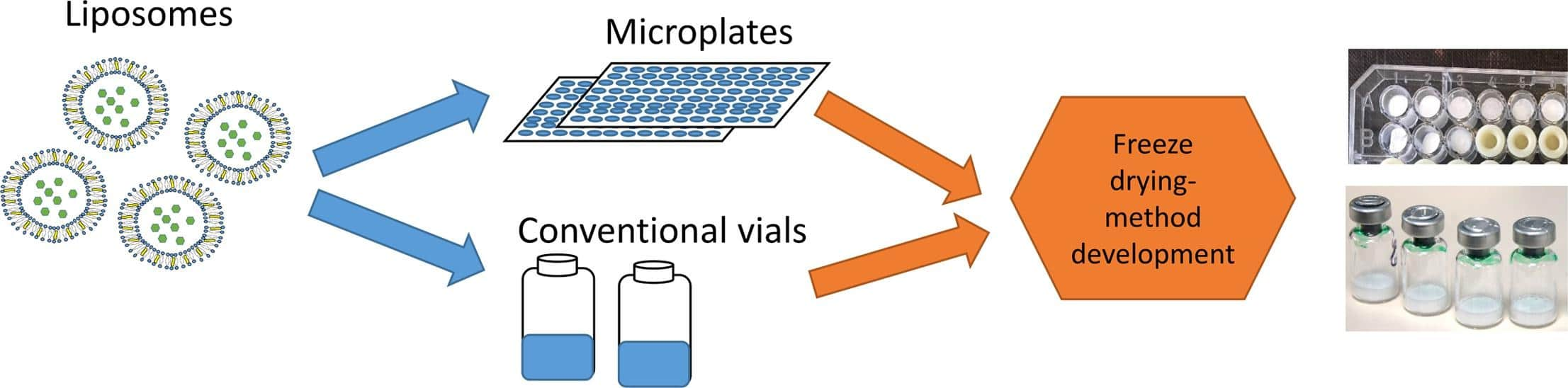 Fig.1 High-throughput screening process using microplate technology to evaluate liposome formulations. (A, Maryam T, et al., 2020)
Fig.1 High-throughput screening process using microplate technology to evaluate liposome formulations. (A, Maryam T, et al., 2020)
The comprehensive and professional evaluation services provided by CD Formulation are backed by advanced technology and equipment. If you have any specific requirements, please feel free to contact us. Our team is here to assist you with consultations.
References
- A, Maryam T. Hussain, et al. Freeze-drying cycle optimization for the rapid preservation of protein-loaded liposomal formulations. International Journal of Pharmaceutics. 2020, 573, 5: 118722
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services




