Lyoprotectant Screening for Liposome Formulation
Inquiry
Due to the potential destabilizing effects of the liposome system during lyophilization, liposomes cannot stably survive freezing damage and dehydration instability. To address this issue, lyoprotectants have been used as specific excipients for liposome formulations. At CD Formulation, our lyophilization research center has dedicated numerous years of effort towards assisting customers in resolving any challenges encountered during the development process of lyophilized liposome formulations, particularly with regard to screening suitable lyoprotectants for liposome formulation. We have been working hard for many years to help customers solve any problems in the development process of lyophilized liposome formulations, especially lyoprotectant screening for liposome formulation. Our team of expert scientists is capable of devising comprehensive and diverse solutions tailored to meet your project requirements.
The Importance of Lyoprotectant Screening for Liposome Formulation
Liposomes are not lyophilized as easily as traditional chemicals, due to the risk of collapse in their vesicle structure caused by dehydration, resulting in a complete loss of activity. Therefore, advanced freeze-drying methods and the addition of specific natural protective additives, known as lyoprotectants, are necessary to safeguard liposomes from these stresses during lyophilization and maintain their physical and chemical properties as well as biological activities. Currently, sugars are considered as the optimal choice for liposome lyoprotectants due to their ability to form both amorphous and crystalline structures that exhibit relative chemical stability while influencing important parameters such as glass transition temperature (Tg' and Tg) during preparation optimization. Moreover, sugars also enhance the redispersibility of lyocakes. Henceforth, the selection of suitable lyoprotectants for liposomes and compatibility studies with other excipients have become increasingly crucial in the development of liposomal formulations.
Lyoprotectant Screening Services for Liposome Formulation
At CD Formulation, we offer you a complete range of lyophilized protective agent screening services. The service mainly matches the appropriate low-temperature protectant system according to the characteristics of different active substances, initially screens out the suitable system, and then evaluates the combination of different systems with different proportions of freeze-dried protectant, and finally screens out the best protective agent formula for you.
We offer the following systems for you to choose from according to the types of lyophilized protective agents commonly used in liposomes, including but not limited to:
- Monosaccharides, including glucose, fructose, and mannose.
- Disaccharides, including, maltose, sucrose, and lactose.
- Three polyols, including mannitol, sorbitol, and glycerol.
- Polysaccharides, including dextran, HP-β, CD cyclodextrin, and soluble starch.
- Amino acids, including sodium glutamate, L-cysteine hydrochloride, histidine, and alanine.
At the same time, we will examine the synergistic or antagonistic effects between lyophilized protectants and buffer salts. For example, two inorganic salts, CaCl2 and MgSO4, can delay the crystallization of trehalose and inhibit crystallization through hydrogen bond networks and changes in molecular mobility in the presence of phosphate anions.
Importantly, our service model is flexible and diverse, according to the characteristics of your project and your requirements, for you to customize the most suitable for your service model.
General Workflow of Lyoprotectant Screening for Liposome Formulation Services
- In the preliminary screening stage, we will provide you with different systems according to different types of lyoprotectants, and ultimately select a more suitable type through matching API and system.
- At the proportion screening stage, the ratio of lyoprotectant is determined and the optimal parameters are obtained based on the mass ratio to lipid.
- At excipent compatibility stage, different types of lyoprotectant combinations are analyzed and compatibility studies are conducted to confirm the synergistic effect between excipients.
- After obtaining the optimal formulation, a comparison is made between the parameters before and after lyophilization. The characteristics of lyocake, particularly stability, zeta potential, particle size, encapsulation rate, etc., are systematically evaluated to provide you with professional and comprehensive data analysis results.
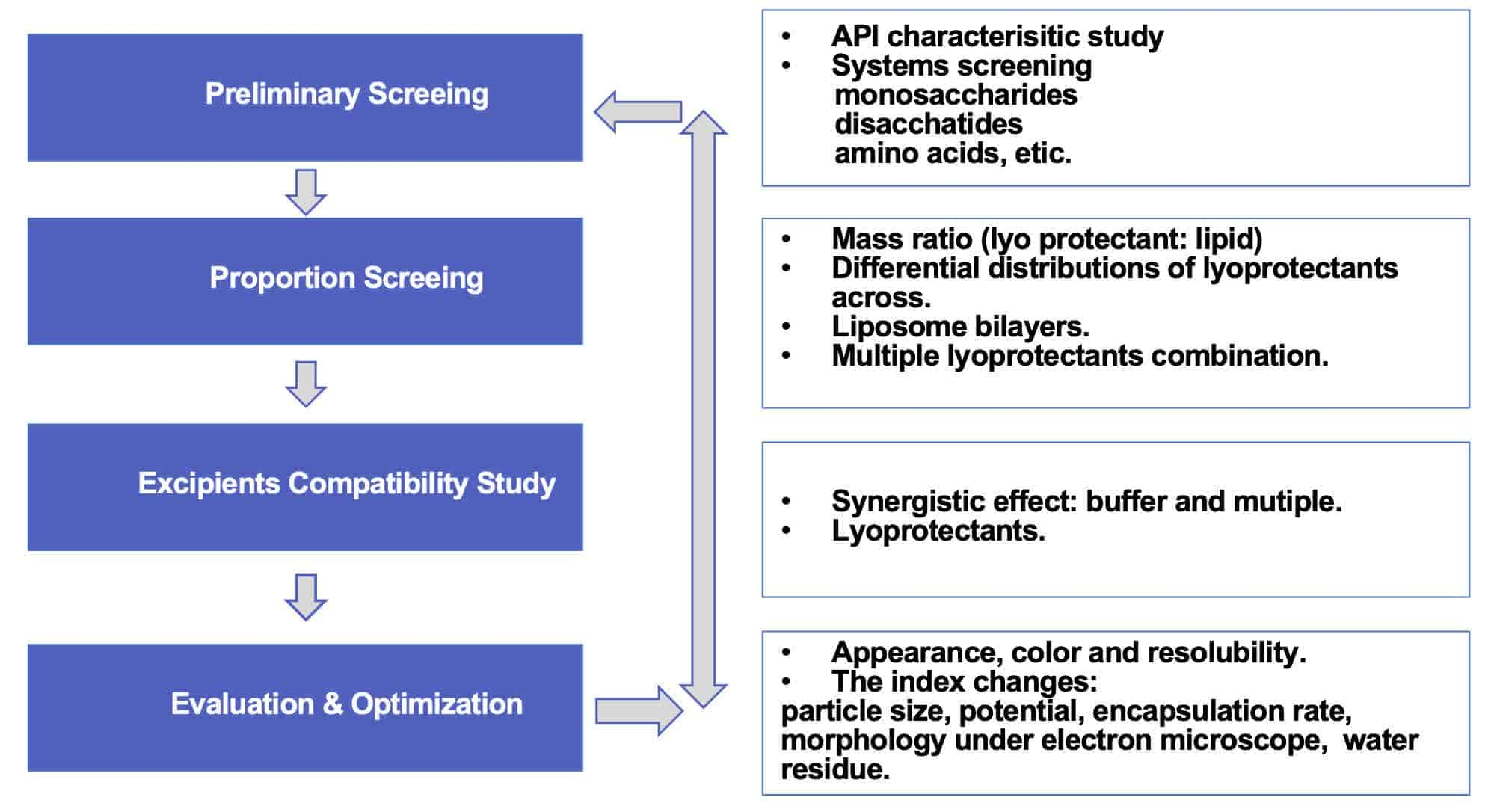 Fig 1 The workflow of lyoprotectant screening for liposome formulation (CD Formulation)
Fig 1 The workflow of lyoprotectant screening for liposome formulation (CD Formulation)
Why Choose Us?
- Expertise: Our team of scientists has extensive experience in lyophilized liposome development, ensuring high-quality, innovative, multiple variants.
- Customization: We offer personalized solutions tailored to the unique needs and requirements of each customer.
- Cutting-edge technology: We utilize advanced technologies to design and optimize the development of liposome lyophilization.
- Quality assurance: We adhere to strict quality standards and conduct comprehensive and systematic testing and evaluation to ensure the safety and efficacy of the liposomes.
Published Data
Technology: Freeze-Drying Technology of Receptor-Targeted Trojan Horse Liposomes
Journal: Molecular Pharmaceutics
IF: 4.321
Published: 2020
Results: In this study, the author developed a suitable freeze-drying method for THL, which effectively preserves its structural integrity and functional properties throughout the freeze-drying/rehydration process. The author conducted a comprehensive screening of various freeze-drying protectants (including trehalose, hyaluronic acid, γ-cyclodextrin, or sulfobutyl β-cyclodextrin) by evaluating plasmid DNA leakage in liposomes, ultimately selecting the optimal freeze-drying protectant formulation. Gene expression was successfully validated post-freeze-drying and rehydration, demonstrating excellent stability over a period of 6 months. Furthermore, in vivo experiments provided compelling evidence supporting the safety and efficacy of the resulting freeze-dried THL.
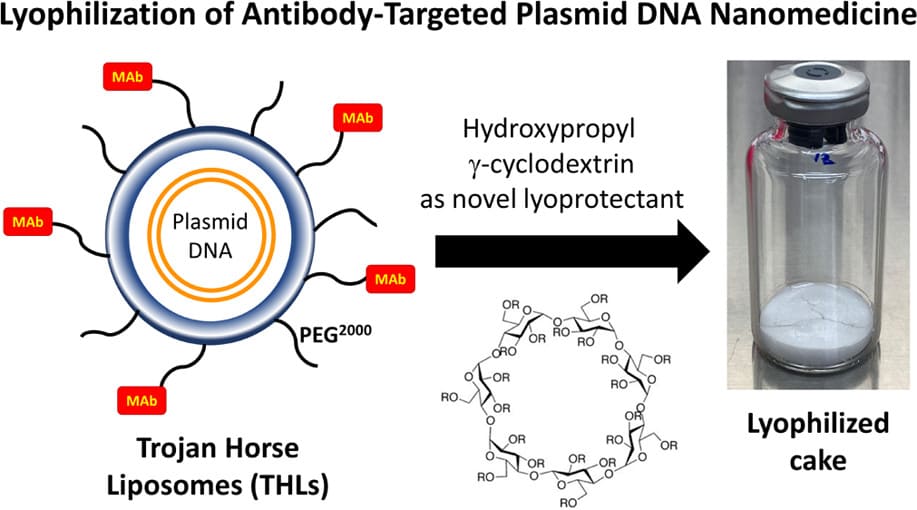 Fig.2 Lyophilization of Antibody-Targeted Plasmid DNA Nanomedicine. (Hungyen Lee, et al., 2024)
Fig.2 Lyophilization of Antibody-Targeted Plasmid DNA Nanomedicine. (Hungyen Lee, et al., 2024)
CD Formulation offers a comprehensive suite of lyoprotectant screening for liposome formulation services. If you are interested in our services or require more details, please feel free to contact us.
References
- Wang Y, Grainger DW. Lyophilized liposome-based parenteral drug development: Reviewing complex product design strategies and current regulatory environments. Adv Drug Deliv Rev. 2019, 151-152: 56-71.
- Hungyen Lee, Dahai Jiang, et al, Lyoprotectant Optimization for the Freeze-Drying of Receptor-Targeted Trojan Horse Liposomes for Plasmid DNA Delivery. Molecular Pharmaceutics.2020, 17 (6): 2165-2174.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig 1 The workflow of lyoprotectant screening for liposome formulation (CD Formulation)
Fig 1 The workflow of lyoprotectant screening for liposome formulation (CD Formulation) Fig.2 Lyophilization of Antibody-Targeted Plasmid DNA Nanomedicine. (Hungyen Lee, et al., 2024)
Fig.2 Lyophilization of Antibody-Targeted Plasmid DNA Nanomedicine. (Hungyen Lee, et al., 2024)
