Liposome Active Loading Technology
Inquiry
Liposomes can be loaded with drugs in two ways: active loading and passive loading. Lipophilic drugs are typically passively loaded, whereas hydrophilic drugs are actively loaded. In the process of industrialization, active loading is often employed to encapsulate drugs in liposomes for ensuring the release and stability of the liposome product. CD Formulation has developed numerous active loading technology platforms to provide tailored solutions for researchers.
What is Liposome Active Drug Loading Technology?
The drug molecules are encapsulated within pre-formed liposomes using the active loading technique, wherein the liposomes internally contain a specific amount of buffer or salt solution. This approach facilitates unidirectional diffusion of drug molecules, resulting in minimal drug loss and, in most instances, achieving an optimal drug-lipid ratio.
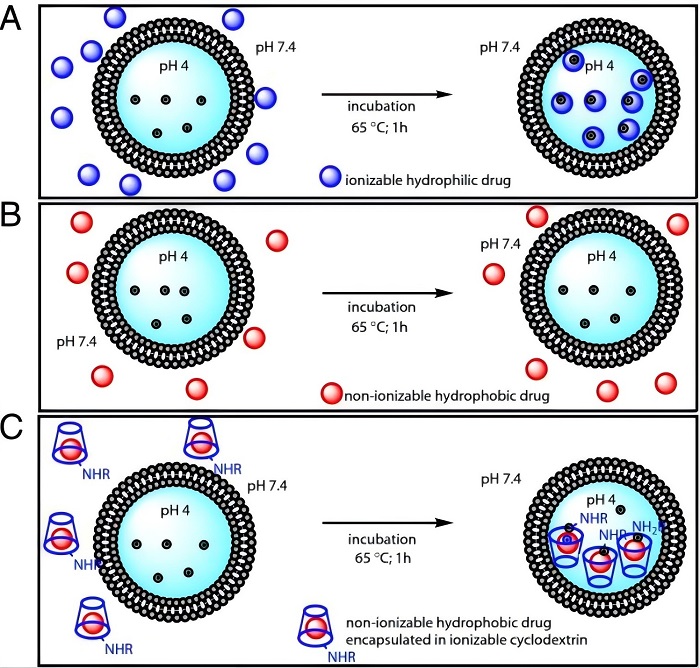 Fig.1 Flowchart of liposome active drug loading. (Sur S, et al, 2014)
Fig.1 Flowchart of liposome active drug loading. (Sur S, et al, 2014)
Our Liposome Active Loading Technology
CD Formulation employs techniques such as investigating the trans-membrane pH gradient, determining suitable incubation time and temperature, selecting appropriate exogenous pH modifiers for the aqueous phase, regulating the buffering capacity of the intracellular aqueous phase, adjusting lipid composition and proportion, etc., to choose the safest and most gentle active drug ion gradient. This enhances liposome encapsulation efficiency and provides a robust foundation for industrializing active drug-loading liposome formulations.
Liposome pH Gradient Technology
This technology primarily adjusts the acidity and basicity of the lipid bilayer's intracellular and extracellular aqueous phases to establish a trans-membrane pH gradient difference and utilizes the variation in dissociation states of weak acidic or basic drugs under different pH environments to enable the drugs to exist in a molecular low-polarity state in the external aqueous phase.
Ammonium Sulfate Gradient Method
This method, specifically applicable to weakly basic drugs with amphoteric properties, such as Adriamycin, Epirubicin, Daunorubicin, and Mitoxantrone, is particularly useful for tumor drugs. The mechanism of action of this method is relatively complex. We have conducted in-depth studies on the mechanics of this technology over the years and have delivered tailored solutions for diverse clients.
Calcium Acetate Gradient Method
The transmembrane calcium acetate concentration gradient is formed during the preparation of liposomes, which promotes the weak acid drug to enter the liposomes and accumulate in the internal water phase. We are very familiar with the preparation process of this technology, which is intended to provide professional support for loading and aggregating weak acidic drugs utilizing this technology.
Advantages of Our Liposome Active Loading Technology
- Diverse Administration Routes for Research Applications: Our sophisticated drug delivery R&D services accommodate a multitude of administration pathways, including oral and injectable forms. Suitable for local applications and subcutaneous injections, our technology broadens the scope of pharmaceutical options, providing a versatile toolkit for researchers. It has been extensively tested and is ready for application in a variety of drug therapies within a research context.
- Optimized Drug Delivery and Release Efficiency: Our drug delivery technology platform is designed to enhance the precision of drug delivery and release. By establishing specific administration routes and active drug delivery methods for each medication, we reduce dosing frequency and enhance bioavailability, offering a valuable asset for pharmaceutical research and development.
- Customizable Design and Characterization for Medications: Our technology is adaptable to various medication types to fulfill the needs of personalized treatment approaches. It improves drug stability, bioavailability, and extends the duration of action, minimizing the challenges associated with frequent administrations. This technology also enables more precise and controlled targeted delivery, showing great promise in specialized research areas, such as oncology.
Explore Our Services Involving Active Loading of Liposomes
Universal Liposome Characterization
The physicochemical properties of liposomes can influence the performance of liposomal drugs, encompassing aspects such as liposome morphology, surface characteristics, structure and integrity, charge distribution, drug encapsulation efficiency, phase transition temperature, particle size distribution, etc.
Liposome Drug Loading Testing
We screen suitable methods for the separation of free liposomes, empty loaded and drug-coated stable liposomes. We provide this service to help our customers determine the exact load and optimize the analysis of the load based on the pharmacodynamic assessment of the different load.
Liposome Drug Delivery System Development
Our cutting-edge liposome technology holds promising applications in various drug delivery fields, such as gene therapy, vaccines, anticancer drugs, and ophthalmic formulations. Through precise control of the structure and composition of liposomes, we can achieve efficient and stable delivery of the active payload.
Published Data
Technology: pH gradient remote loading technique
Journal: Acta Pharmaceutica Sinica B
IF: 14.7
Published: 2021
Results: This study designed a series of weak acid drug derivatives through a simple one-step synthesis and loaded them into liposomes using a pH gradient method. A weak acid carboplatin derivative (CTX) was selected for safety, pharmacokinetics, and pharmacodynamic evaluation. In terms of safety, the CTX-loaded weak acid derivative liposome performed better than Jevtana®, demonstrating superior systemic toxicity, hematological toxicity, and potential central nervous system toxicity mitigation. Specifically, the liposome was able to mitigate the potential toxicity of CTX to cortical and hippocampal neurons. Due to the high safety profile and tolerable dosage of CTX-loaded weak acid derivative liposomes, they offer significant advantages in the treatment of prostate cancer and metastatic cancer.
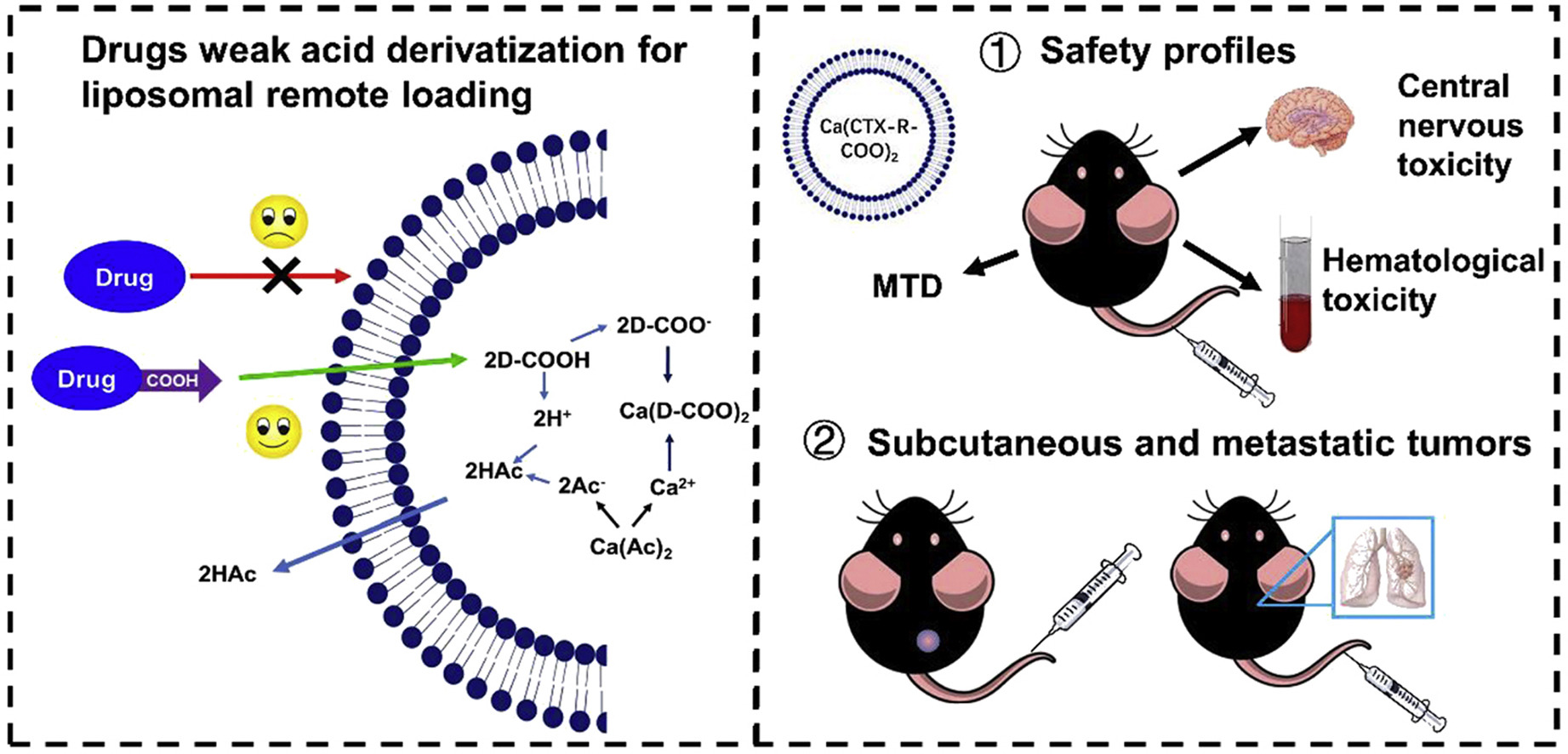 Fig.2 Liposome-based drug delivery system for non-ionic compounds. (Zhou S, et al., 2021)
Fig.2 Liposome-based drug delivery system for non-ionic compounds. (Zhou S, et al., 2021)
As a leader in liposome formulations, CD Formulation offers specialized contract R&D services for liposome active drug loading. Please feel free to contact us if you need any assistance.
References
- Sur S, Fries AC, et al. Remote loading of preencapsulated drugs into stealth liposomes. Proc Natl Acad Sci U S A. 2014 Feb 11; 111(6): 2283-8.
-
Zhou S, Li J, et al. A facile and universal method to achieve liposomal remote loading of non-ionizable drugs with outstanding safety profiles and therapeutic effect. Acta Pharmaceutica Sinica B. 2021 Jan 1; 11(1): 258-70.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.

 Fig.1 Flowchart of liposome active drug loading. (Sur S, et al, 2014)
Fig.1 Flowchart of liposome active drug loading. (Sur S, et al, 2014) Fig.2 Liposome-based drug delivery system for non-ionic compounds. (Zhou S, et al., 2021)
Fig.2 Liposome-based drug delivery system for non-ionic compounds. (Zhou S, et al., 2021)
