NGS-Based Adeno-Associated Virus Quality Testing
Inquiry
Next-generation sequencing (NGS)-based adeno-associated virus (AAV) quality control solution brings new opportunities to improve the quality of gene therapy products due to its high throughput and high sensitivity. NGS-based AAV quality control services have significant application prospects and value as they can effectively characterize viral vectors in depth and ensure the safety and efficacy of gene therapy products.
CD Formulation provides advanced technical support and perfect solutions for gene therapy research. We continue to improve and optimize NGS-based AAV quality testing solutions, aiming to provide more comprehensive and reliable technical support for quality control and therapeutic efficacy assessment of gene therapy products.
Importance of NGS-Based AAV Quality Testing
- AAV sequence analysis. NGS technology allows whole genome sequencing or targeted region sequencing of AAV samples to quickly and accurately determine the viral genome sequence and identify potential sequence variants. This helps assess the purity, consistency and stability of AAV products.
- Integration site analysis. Integration of AAV gene therapy products into the host genome may raise potential safety concerns, and therefore, integration site analysis is critical for evaluating the safety of therapeutic products. NGS technology can efficiently detect AAV integration sites and accurately identify relevant sequences in the host genome.
- Impurity detection. Impurities in AAV products may affect therapeutic efficacy and safety, and NGS technology enables comprehensive contaminant detection and impurity analysis of AAV samples, including exogenous DNA, bacteria, and fungi.
Our Services for NGS-Based AAV Quality Testing
AAV sequence analysis
We utilize NGS technology to perform whole genome sequencing or target region sequencing of AAV samples to ensure the accuracy of the viral genome sequence and to assess product purity, consistency and stability.
AAV integration site analysis
We apply NGS technology to detect AAV integration sites and identify relevant sequences in the host genome, providing important data for the safety assessment of therapeutic products.
AAV impurity testing
We conduct comprehensive contaminant and impurity analyses of AAV samples, including exogenous DNA, bacteria, fungi, etc. We utilize NGS to ensure the purity and quality of our products.
Specialty sample testing
We can also provide several NGS sequencing-based testing services for unknown or complex samples, including capsid protein identification, host cell DNA residues, plasmid DNA residues, and more.
Overall, AAV is being widely researched and developed as a vector for gene therapy. The application of NGS technology can improve the design of AAV vectors and greatly enhance the efficiency and safety of AAV in gene therapy.
Our Platforms for AAV Quality Testing
| Platforms & Technologies |
Content Description |
| Analytical ultracentrifugation (AUC) |
This is a gold-standard method for determining the proportion of different forms of viral particles in AAV samples, allowing precise characterization of the individual components of the sample, including both empty capsid and solid viral particles. |
| High-performance liquid chromatography-mass spectrometry (HPLC-MS) |
This technique ensures complete sequence coverage of AAV by lysing AAV, digesting AAV capsid proteins enzymatically into peptides and then detecting them by HPLC-MS/MS, which can be used for molecular weight and ratio analysis of AAV capsid proteins and peptide mapping of AAV capsids. |
| Digital PCR (dPCR) |
Used to detect genomic titers in AAV samples, providing the expected titer range, primer-probe sequence, and sample volume for a single test. |
Highlights of Our NGS-Based AAV Quality Testing
- Comprehensive quality testing. Using NGS technology, we can perform comprehensive contaminant detection and impurity analysis on AAV samples, providing comprehensive quality control for gene therapy products.
- Personalized and customized services. We offer customized solutions to meet a wide range of research needs based on our client's specific requirements.
- Rapid response. We are committed to providing fast and efficient sequencing services to minimize your waiting time.
- Complete service process. We can provide a one-stop service from gene synthesis, and vector construction to virus packaging.
Published Data
Technology: High-throughput sequencing assay technology
Journal: Biotechnol J
IF: 5.7
Published: 2021
Adeno-associated virus (AAV) has important applications in the field of gene therapy, and the safety of AAV-based therapies requires the creation of quality control tests, especially to check for unwanted DNA sequences that may be harmful. A new high-throughput sequencing (HTS) assay has been developed to measure residual DNA in AAV formulations. Here, it is shown that certain DNA regions with high GC content and repetitive sequences can cause sequencing errors. To fix this, a new PCR-free technique was introduced, which provides more uniform coverage and better accuracy in measuring residual DNA. This HTS approach is more thorough than traditional real-time PCR tests and helps enhance the safety and effectiveness of AAV as a therapeutic tool.
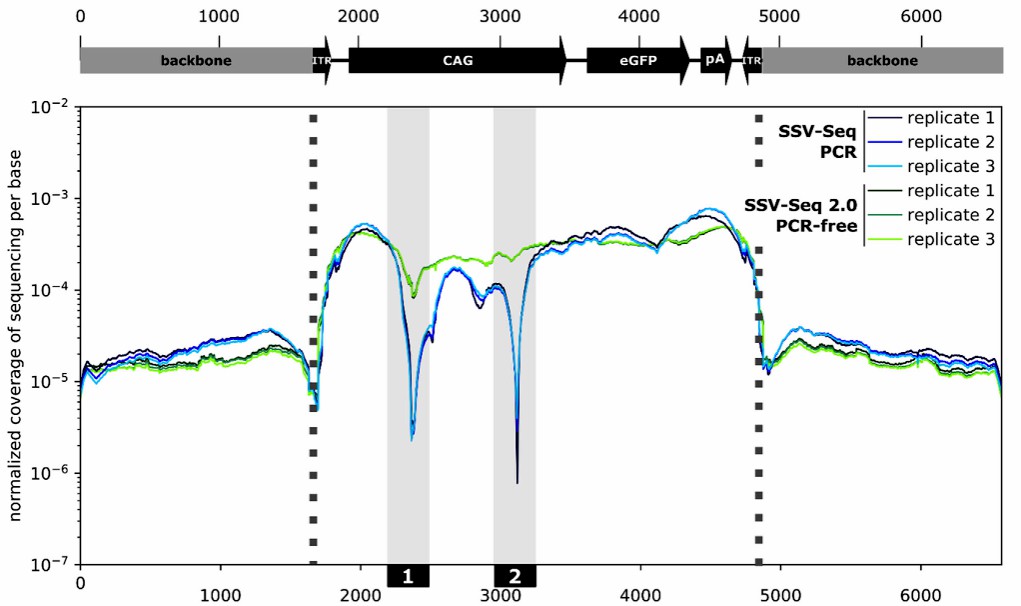 Fig.2 Sequencing coverage along the adeno-associated virus vector genome and the plasmid backbone. (Lecomte E, et al., 2021)
Fig.2 Sequencing coverage along the adeno-associated virus vector genome and the plasmid backbone. (Lecomte E, et al., 2021)
With the continuous development and application of NGS technology, CD Formulation aims to provide important technical support to improve the quality and therapeutic effect of gene therapy products through the NGS-based QC program, which will bring broader prospects for the development and application of the gene therapy field. If you are interested in us, please feel free to contact us.
References
- Lecomte E, et al. The SSV-Seq 2.0 PCR-Free Method Improves the Sequencing of Adeno-Associated Viral Vector Genomes Containing GC-Rich Regions and Homopolymers. Biotechnol J. 2021, 16(1):e2000016.
Related Services

 Fig.2 Sequencing coverage along the adeno-associated virus vector genome and the plasmid backbone. (Lecomte E, et al., 2021)
Fig.2 Sequencing coverage along the adeno-associated virus vector genome and the plasmid backbone. (Lecomte E, et al., 2021)