Long-Read Sequencing for Adeno-Associated Virus Gene Therapy
Inquiry
Recombinant AAV (rAAV) is one of the most common vectors for gene therapy. In the most basic form of this virus, the rAAV vector has been stripped of all its components, leaving only the flanking inverted terminal repeat (ITR) region, which is used to guide genome replication and packaging during vector production. CD Formulation provides comprehensive technical support and reliable solutions for gene therapy research. We provide long-read sequencing technology services to address the problems of short-read sequencing technology in gene therapy and contribute to promoting gene therapy research.
Technical Advantages of Long-Read Sequencing for AAV Gene Therapy
- High sequencing accuracy. The ability to generate long and accurate read sequences with high accuracy helps ensure that AAV vectors used in gene therapy contain the correct therapeutic gene sequences.
- Full-length AAV genome sequencing. The entire AAV genome can be sequenced, including the ITR inverted terminal repeat region and high GC content regions.
- Improved vector design. Previously invisible errors in vector design can be revealed, such as whether the insert sequence of the gene therapy was kept correct during development and whether structural regions of the gene therapy need to be improved.
- Quality control. Contributes to strict quality control of AAV vector products, including testing the purity, homogeneity, and fidelity of the packaged DNA to ensure the safety and efficacy of the gene therapy.
Our Long-Read Sequencing for AAV Gene Therapy
Long-read sequencing for AAV vector discovery
We offer long-read sequencing technology that provides highly accurate long-read sequences, which are critical for the discovery of novel AAV capsids. At the same time, long-read sequencing technology enables us to read DNA sequences that are thousands or even tens of thousands of bases long, helping researchers to comprehensively analyze the genome structure of AAV, including their gene modules and the complex repeats within them.
Long-read sequencing for vector design
Long-read sequencing plays a key role in AAV vector design, revealing packaging issues and genomic truncations. For example, we have used this sequencing technology to discover the actual full-length sequences in AAV vector clusters, which can help improve vector design and enhance the safety and efficacy of gene therapy by designing AAV vectors with better gene chimerization and splicing to meet specific therapeutic needs.
Long-read sequencing for host integration studies
For AAV vector integration studies in the host, long-read sequencing technology can provide fine molecular-level details to completely capture the location, integration frequency, integration mode and so on of AAV vectors integrated in the host genome. This comprehensive genomic data helps to understand the mechanism of AAV vector integration, assess the potential genomic instability and oncogenic risk associated with its integration, and improve vector design and safety of use.
Long-read sequencing for AAV production
During AAV production, long-read sequencing technology is mainly used for quality control and optimization of the production process. Examples include monitoring the integrity and purity of the AAV genome to ensure the absence of unintended recombinants or contamination, as well as ensuring production consistency and product quality. In addition, long-read sequencing is also used to optimize AAV production cell lines by fully resolving the genomes of the production cells and precisely adjusting and improving the cell lines to produce high-quality AAV virus particles more efficiently and safely.
Our Platforms for AAV Gene Therapy Long-Read Sequencing
| Platforms & Technologies |
Content Description |
| HiFi sequencing technology |
A single-molecule real-time (SMRT) sequencing technology capable of generating long read lengths of up to 20,000 base pairs, making possible the complete sequencing of the AAV genome. HiFi sequencing can be used in the discovery and design of AAV vectors, host integration studies, and the production process, and has revealed issues that are difficult to detect with other methods, such as the novel discovery of AAV capsids, improved vector design, and understanding of host integration events, as well as comparative analyses of production platforms. improvement of vector design, understanding of host integration events, and comparative analysis of production platforms. |
| Nanopore sequencing technology |
Known for its ultra-long read lengths, this technology enables real-time, long-read detection of individual DNA molecules and is suitable for macro-genomics studies and sequencing of complex genomic regions. For example, in AAV gene therapy, Nanopore technology is used to reveal the high-resolution integration of HPV in cervical cancer and its oncogenic progression. |
| Synthetic long-read sequencing technology |
This is a method that combines short-read long sequencing and specific markers to combine short-read long sequencing results with long-read sequencing results by adding special markers during the interruption of long segments of DNA. This technology helps to improve the resolution of genomic structural variation detection. |
Highlights Our Long-Read Sequencing for AAV Gene Therapy
- Advanced synthesis technology. We can synthesize difficult repeat sequences, difficult sequences with high GC content, and ITR regions containing hairpin structures.
- Reliable quality. We provide dual testing of digestion and sequencing, and our ITR sequencing technology is capable of sequencing through the ITR region to ensure that the sequence is 100% correct.
- Free expression system optimization. We can codon optimize the target gene according to the expression system.
- Short cycle time. Our advanced technology platform and experienced team of experts ensure the fastest delivery of results for you.
Published Data
Technology: Nanopore sequencing technology
Journal: Hum Gene Ther
IF: 3.9
Published: 2022
Recombinant adeno-associated viruses (rAAVs) are key in gene therapy due to their efficiency in delivering genetic material into cells. However, the quality of these vectors can be compromised by production methods that result in a mix of different types. Recent advancements in sequencing technology, particularly long-read methods like SMRT, have improved the analysis of vector diversity but are not always accessible or practical for all researchers. Nanopore sequencing offers a faster and more affordable alternative for long-read sequencing. This study tested nanopore sequencing's ability to analyze rAAVs and found it could sequence entire genomes without pre-fragmentation, although with some inaccuracies in base calling. Despite these limitations, nanopore sequencing successfully identified important vector features, suggesting it's a viable, albeit imperfect, tool for quickly assessing rAAV vector quality.
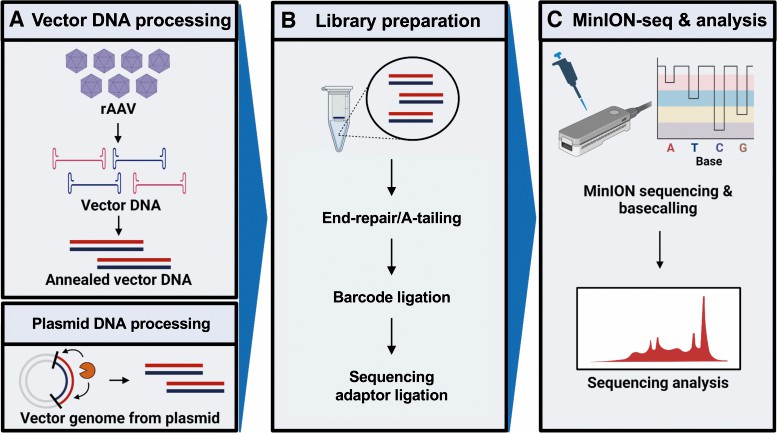 Fig.2 Workflow for nanopore sequencing and analyses of adeno-associated viruses. (Namkung S, et al., 2022)
Fig.2 Workflow for nanopore sequencing and analyses of adeno-associated viruses. (Namkung S, et al., 2022)
CD Formulation offers comprehensive solutions for every stage of gene therapy research and development. From target discovery to scale-up, we provide rigorous technical support throughout our clients' projects. If you are interested in us, please feel free to contact us.
References
- Namkung S, et al. Direct ITR-to-ITR Nanopore Sequencing of AAV Vector Genomes. Hum Gene Ther. 2022, 33(21-22):1187-1196.
Related Services


 Fig.2 Workflow for nanopore sequencing and analyses of adeno-associated viruses. (Namkung S, et al., 2022)
Fig.2 Workflow for nanopore sequencing and analyses of adeno-associated viruses. (Namkung S, et al., 2022)