Gene Delivery Systems for Gene Therapy Formulation Development
Inquiry
Gene delivery systems are a central component in gene therapy, enabling efficient delivery of gene fragments such as plasmid DNA, mRNA, and proteins into cells. The efficiency of this process is often positively correlated with the success rate of gene editing. CD Formulation has many years of research and service experience in gene delivery system development and is committed to providing advanced and reliable gene delivery system development services, including viral delivery development and non-viral delivery system development.
Importance of Gene Delivery Systems Development
- Targeted delivery of gene therapy drugs. Gene drugs are easily degraded in the body, and the development of an effective gene delivery system can precisely deliver gene drugs to target tissues, target cells, and target organelles so that they can fully play their roles.
- Reduce off-target editing. The development of gene delivery systems can reduce the exposure time of gene therapy drugs and minimize the possibility of off-target editing.
- Reduce immunogenicity. Some gene delivery vectors may trigger an immune response, affecting therapeutic efficacy. Developing delivery systems with low immunogenicity can reduce the occurrence of immune reactions and improve the safety of gene therapy formulations.
- Provide technical support. The development of gene delivery systems provides key technical support for the research and development of gene therapy formulations and plays an important role in promoting the innovation and research of gene therapy formulations.
Explore Our Gene Delivery Systems Development
We are committed to providing comprehensive and reliable gene delivery system development services to support the development and optimization of gene therapy formulations. We continue to optimize our technology systems and provide an extensive gene therapy formulation development technology platform that meets stringent regulations to create reliable, effective, safe, and durable gene delivery vectors to help researchers fuel progress in the development of gene therapy formulations.
Viral Gene Delivery Systems Development Service
Our high-quality viral vector development services include the provision of a wide range of viral vector development not limited to adeno-associated viral vectors, adenoviral vectors, lentiviral vectors, retroviral vectors, etc., and our services encompass a comprehensive range of viral vector development services not limited to the viral vectors that we have listed above. It is important to note that we can also provide customized development services according to the characteristics and requirements of renewable projects to best meet the research needs of our clients.
Non-Viral Gene Delivery Systems Development Service
We have a quality track record in providing non-viral vector delivery system development, including but not limited to providing a reliable solution to possible safety, immune response, and other issues with viral vectors. We continually optimize and update our non-viral delivery vector development services to develop and provide high-quality, safe non-viral vectors. Our current viral vector development services include but are not limited to, naked DNA development, liposome-mediated gene delivery development, exosome delivery vehicles development, nanoparticle delivery system development, cell-penetrating peptide delivery system development, bacterial vector development, polymeric gene carriers, stem cell vehicle development. Our comprehensive non-viral delivery system development services can best meet your multiple drug delivery needs in gene therapy formulation development.
Our Process of Gene Delivery Systems Development
- Selection and optimization of vectors. According to the project requirements and vector characteristics, determine the appropriate vector, and consider its delivery efficiency, cytotoxicity, immunogenicity and other factors in the selection of the carrier.
- Delivery system design. This part includes the design of expression vectors, i.e., the design of expression vectors containing the target genes to ensure that the target genes can be efficiently expressed in the target cells. Subsequently, it is necessary to optimize the composition and preparation method of the delivery system through experiments to improve delivery efficiency and reduce cytotoxicity.
- In vitro experimental validation. We utilize cellular experiments and gene expression validation in this process for in vitro experimental validation.
- In vivo experimental validation. We use some animal models in this process to perform in vivo gene delivery experiments to evaluate the tissue distribution, gene expression, and immune response of the delivery system.
- Preclinical studies. We mainly perform drug metabolism and toxicity evaluation, as well as comprehensive assessment.
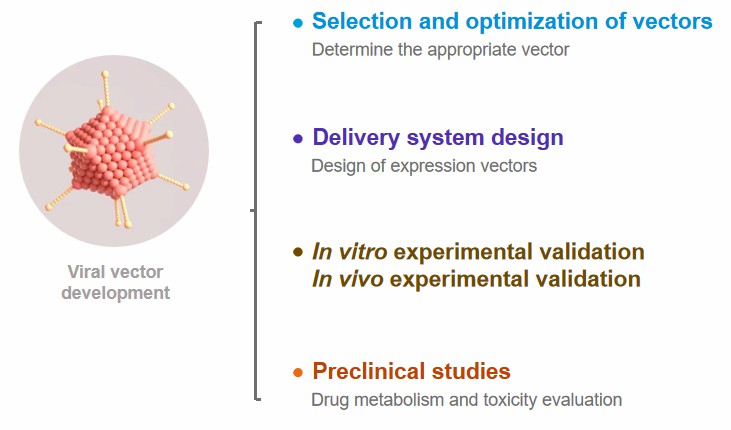 Fig.2 Our process of gene delivery systems development. (CD Formulation)
Fig.2 Our process of gene delivery systems development. (CD Formulation)
Our Platforms for Gene Delivery Systems Development
We have established advanced technology platforms to support gene delivery system development. We have mature gene editing platforms, algorithmic systems for efficient sgRNA and various innovative gene knock-in technologies, as well as vector construction and viral packaging technology platforms, among others. Below is a list of some of the most commonly used technology platforms.
| Technologies & Platforms |
Content Description |
| Virus packaging |
We have established a comprehensive viral packaging technology platform that can effectively improve the efficiency and safety of gene delivery and provide the possibility of long-term stabilization of gene expression. Our advanced viral packaging technology platform supports the packaging of a wide range of viral vectors including lentivirus, adeno-associated virus, VSV virus, and retrovirus. |
| Vector construction |
Our advanced vector construction technology platform provides hundreds of vector systems optimized for gene therapy agent development and application, all validated in vitro and in vivo. Our technology platform allows for vector customization and cloning and significantly improves research efficiency. |
| CRISPR/Cas9 technology |
CRISPR/Cas9 can be used to construct viral vectors to insert or delete specific sequences through precise gene editing. Based on gene editing as a technology platform, we can help researchers customize therapeutic genes and integrate them into viral vectors. |
Highlights of Our Gene Delivery Systems Development
- We always uphold the concept of efficiency and safety, continue to optimize and streamline the development process, improve productivity with cutting-edge technology, effectively reduce potential risks, and build reliable research support for our customers.
- We are committed to the development of gene delivery vectors and provide customized service solutions to meet the needs of different research objectives.
- We are committed to providing innovative gene delivery vector development services to enhance the success rate of gene expression and assist researchers in the development of gene therapy formulations.
CD Formulation is committed to systematically overcoming the key technological bottlenecks in the gene delivery industry, empowering basic research, and accelerating the development of gene therapy formulations. If you are interested in us, please feel free to contact us.
Related Services


 Fig.2 Our process of gene delivery systems development. (CD Formulation)
Fig.2 Our process of gene delivery systems development. (CD Formulation) 