- Home
- Services
- Antibody Delivery System Development
- Antibody Preparation Services
- Common Antibody Formulation Development
- Antibody Nanoparticle Development
- MOFs Customization for Antibody Delivery
- Antibody Cocktail Therapy Development
- Antibody Complexes Development
- Antibody-Modified Immunoliposome Development
- Antibody Drug Process Development and Scale-up
- Antibody Humanization Service
- Antiserum Preparation Services
- Customized Drug Conjugates Services
- Peptides & Proteins Delivery System Development
- Peptide & Protein Service
- Common Peptide & Protein Formulation Development
- Peptide & Protein Microneedle Array Development
- Peptide & Protein Transdermal Patch Development
- Exosome Development for Peptide & Protein Delivery
- Peptide & Protein Liposome Development
- Peptide & Protein Gold Nanoparticle Development
- Nanoparticle Development for Peptide & Protein Delivery
- Peptide & Protein Microsphere Development
- Hydrogel Development for Peptide & Protein Delivery
- Peptide & Protein Block Copolymer Micelle Development
- Peptide & Protein HA Conjugation System Development
- Computer-Aided Peptide & Protein Formulation Development
- Nucleic Acid Delivery System Development
- Nucleic Acid Vaccine Adjuvant Development
- Immunostimulant Development for Vaccine Delivery
- Nucleic Acid Delivery Excipient Customization
- Nucleic Acid Injection Formulation Development
- Nucleic Acid Microneedle System Development
- Nucleic Acid Mucosal Delivery Formulation Development
- VLP-Based Nucleic Acid Delivery System Development
- Viral Vector Development for Nucleic Acid Delivery
- Cell-Based Vector Development for Nucleic Acid Delivery
- Bacterial-Based Nucleic Acid Delivery System Development
- Nanocarrier Development for Nucleic Acid Delivery
- Hydrogel Development for Nucleic Acid Delivery
- Cell Therapy Development
- One-stop Solutions for Biosimilar Testing
- In Vitro Antiviral Testing Services
- Analytical Testing Services
- Structural Characterization and Confirmation
- Physicochemical Properties
- General Testing Services
- Protein Content Determination
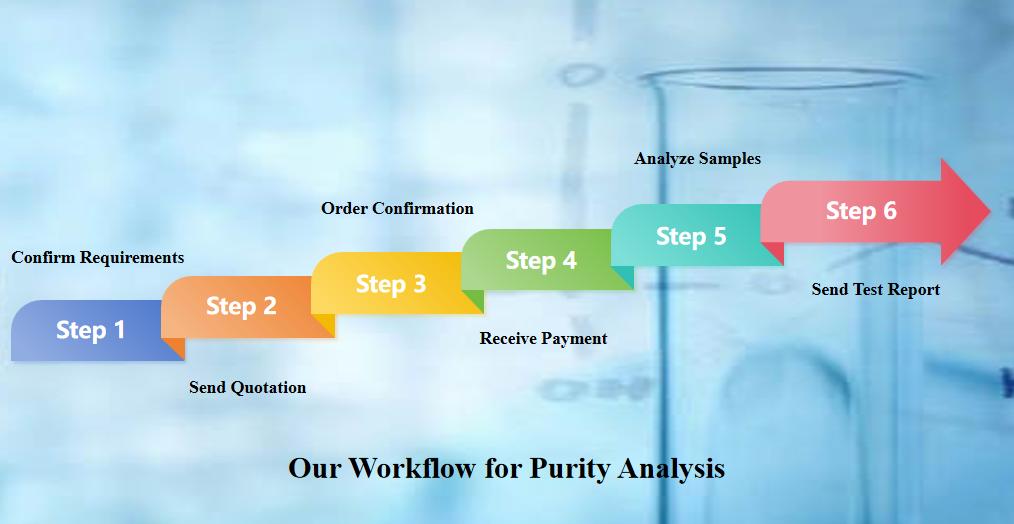
- Purity Analysis Services
- Impurity Determination
- Contaminants Analysis
- Biological Activity (Potency) Testing Services
- Stability Testing
- Analytical Method Validation of Bioactivity and Potency
- One-stop Solutions for Biomacromolecule Drug Formulation Development
- One-stop Solutions for Cell Therapy Product Development
- One-stop Solutions for Nucleic Acid Drug Formulation Development
- One-stop Solutions for Antibody Formulation Development
- One-stop Solutions for Vaccine Formulation Development
- One-stop Solutions for Antibody-Drug Conjugates (ADCs) Formulation Development
- One-stop Solutions for Peptides & Proteins Formulation Development
- One-stop Solutions for Micro-ecological Probiotic Formulation Development
- One-stop Solutions for Biosimilars Development
- Exosome Formulation Services
- Immunology Testing Services
- Culture Medium Services
- Omics Services
- Antibody Drug In Vitro Efficacy Evaluation Services
- Antibody Delivery System Development
- Products
- Online Order
- Company
- Inquiry