Based on an advanced precision instrument detection platform, CD Formulation can perform separation and determination of a variety of protein or peptide samples to meet customers' specific needs for purity analysis of different protein or peptide samples.
Why to Analyze Protein Purity?
Generally, impure protein samples can be detrimental in a number of ways, contributing to experiment inaccuracies or affecting product performance. Protein purity measurement can evaluate whether the protein contains impurities and the content of impurities, and is a crucial step in further protein research and bio-pharmaceutical applications. The purity of protein samples limits the applications of protein products. Therefore, it is necessary to purify them and detect the purification results, that is, protein purity analysis, to ensure product quality and safety.
Our Methods for Protein Purity Analysis
With the development and progress of life science research, methods for measuring protein purity are becoming more and more diverse, CD Formulation can provide protein purity analysis based on the following methods, including Electrophoresis, chromatography (Gel filtration chromatography, reversed phase HPLC), Sedimentation velocity, mass spectrometry, etc.
Among electrophoretic methods, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is commonly used to identify protein purity. SDS-PAGE mainly separates proteins based on the different mobilities generated by the different relative molecular masses of different protein molecules.
Gel filtration chromatography is non-destructive and very fast. Because this is a zonal method.
Reversed-phase HPLC uses a nonpolar matri (such as modified silica media) as the stationary phase. The organic solvent in the mobile phase reduces the affinity between the protein and the stationary phase and allows gradient elution with reduced polarity, allowing the protein to be eluted.
- Sedimentation velocity method
The sedimentation velocity method can determine protein purity simply, quickly, and non-destructively, and is very sensitive to the ratio of molecular mass to molecular size.
Mass spectrometry can directly measure the distribution of covalent mass in a sample.
Measure single samples with static or dynamic light scattering, or monitor HPLC and field-level separation processes. Enhanced ability to identify eluted proteins through continuous determination of molecular size and apparent molecular mass to differentiate between analytes, multimers of analytes, or impurities.
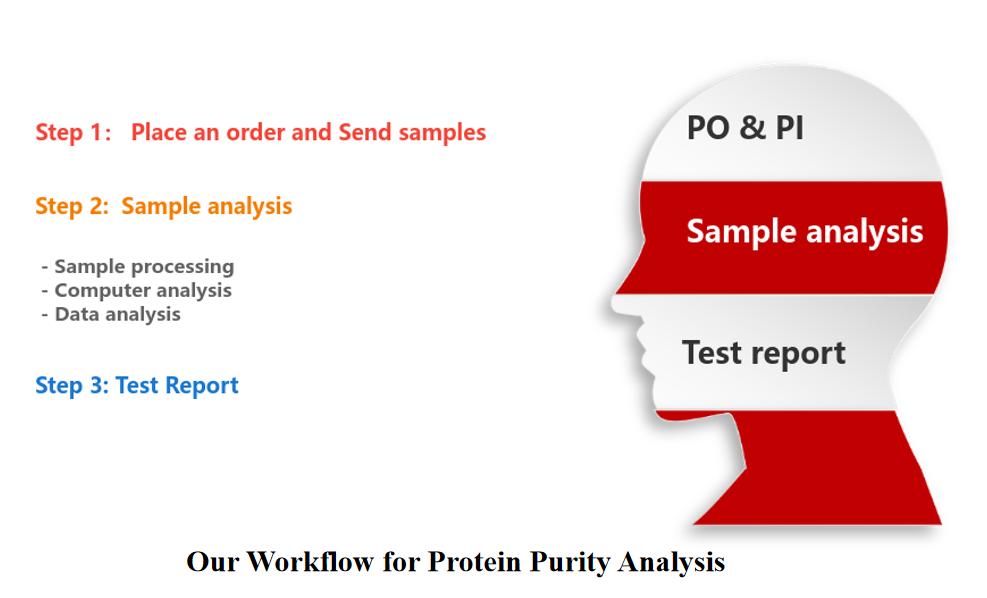
Our Workflow for Protein Purity Analysis
You only need three steps to easily solve your requirements.
Advantages of Our Services
- Advanced technology platforms: We are equipped with multiple technology platforms to accurately determine protein purity and provide accurate and detailed results on sample purity.
- Professional technical team: We have an experienced technical team that can select appropriate methods for protein purity determination based on customer needs to ensure the accuracy and reliability of the results.
- Super clear service process: We have a very clear service process to guide customers quickly and clearly on the progress of the project.
How to Contact us?
If you have a requirement about our services, please contact us by phone or email, our colleagues will reply to you within three working days.
Related Services




