Solid Dispersion Oral Thin Film Preparation Technology
Inquiry
Oral thin film size is determined based on active pharmaceutical ingredient (API) solubility and a required drug dose. High drug loading could negatively affect the physical properties of oral thin films, such as thickness, disintegration time, and mechanical properties, or lead to recrystallization of the amorphous API. During the disintegration of an oral thin film, insoluble particles also could negatively affect the perception in the mouth. When an API with poor solubility is used to prepare an oral thin film, it is first necessary to increase its solubility. Solid dispersion is a technique that improves the solubility and dissolution rate of poorly water-soluble drugs, and it is the most frequently and effectively used one. CD Formulation provides solid dispersion technology to help customers increase the solubility of insoluble APIs, improve the drug load of oral thin films, and help customers develop oral thin films with high drug load.
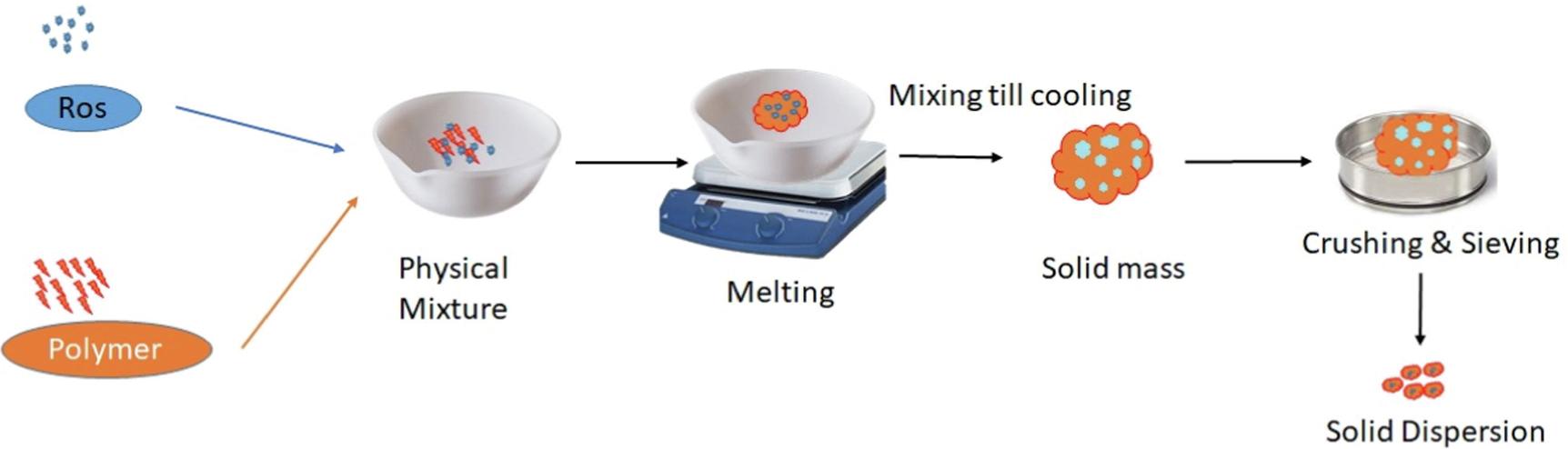 Fig.1 Solid dispersion preparation method. (Randa Mohammed Zaki, et al., 2023)
Fig.1 Solid dispersion preparation method. (Randa Mohammed Zaki, et al., 2023)
Preparation Methods of Solid Dispersion
The methods used to prepare solid dispersion include melting method, solvent method and mechanical dispersion method. CD Formulation relies on our advanced design concept to use the following preparation methods to prepare solid dispersion, improve the dissolution of insoluble APIs, and support the development of oral thin film products.
Melting Method
The melting method is to prepare the drug and the carrier proportionately into a low eutectic or solid solution, and then rapidly cool it to form a solid dispersion. This process often has high requirements for API and carriers, requiring API and carrier to melt simultaneously or API to dissolve in the melted carrier, and the two cannot be degraded.
Solvent Method
The solvent method is the most traditional method for preparing solid dispersion. The carrier and API are dissolved in a benign solvent, and then the solvent is evaporated to obtain a solid dispersion.
Mechanical Dispersion Method
The mechanical dispersion method highly disperses the drug particles in the carrier through strong mechanical energy. The mechanical pulverizer can crush the particle size to less than 10 microns, the airflow pulverizer can crush the particle size to less than 5 microns, and the homogenizer can control the particle size to less than 1 micron.
Our Analytical Capabilities for Solid Dispersion
To characterize and assess the feasibility of the solid dispersions, we can provide you with the following characterization of solid dispersions including:
| Properties |
Methods Used |
| Physical State Examination |
Differential scanning calorimetry
Powder X-ray diffraction
Hot stage microscopy
Humidity stage microscopy |
| Surface Microscopy |
Scanning electron microscopy
Polarized light optical microscopy |
| Structure Elucidation |
Solid state nuclear magnetic resonance spectroscopy
Fourier transform infrared spectroscopy |
| Drug Carrier Interactions |
Differential scanning calorimetry
Nuclear magnetic resonance spectroscopy
Fourier transform infrared spectroscopy |
| Dissolution Rate |
Dissolution studies
Dynamic solubility studies |
| Stability |
Differential scanning calorimetry
Nuclear magnetic resonance spectroscopy
Fourier transform infrared spectroscopy |
Workflow of Oral Thin Film Solid Dispersion Preparation
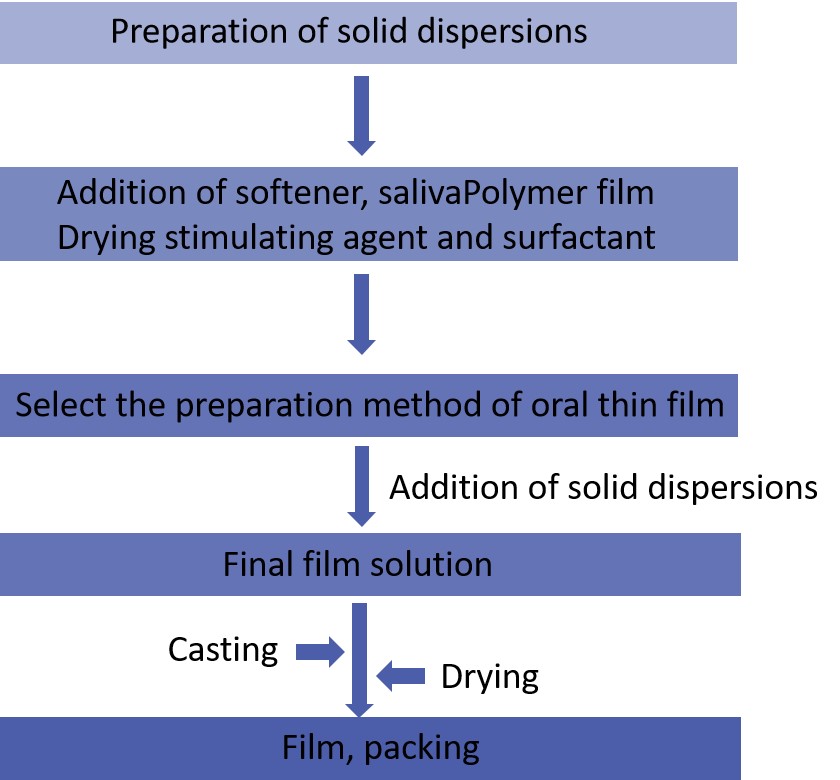 Fig.2 Workflow of Oral Thin Film Solid Dispersion Preparation. (CD Formulation)
Fig.2 Workflow of Oral Thin Film Solid Dispersion Preparation. (CD Formulation)
Explore the Characterization of Solid Dispersion Oral Thin Film
We offer a range of solubilization technologies designed to enhance the solubility and bioavailability of poorly water-soluble drugs, facilitating the development of more effective oral thin film products. At the same time, we provide a range of characterization services for solid dispersion oral thin film.
Characterization of Solid Dispersion Oral Thin Film
The prepared solid dispersion oral thin film is thoroughly characterized to evaluate its physical and chemical properties, including film thickness, uniformity, drug content, mechanical strength, dissolution behavior, etc.
Advantages of Our Solid Dispersion Oral Thin Film Preparation Technology
- We are very experienced in the formulation development of oral thin films containing solid dispersions and can efficiently characterize solid dispersions and oral thin films.
- Our oral thin film solid dispersion preparation technology is designed to significantly improve poorly water-soluble drugs' solubility and dissolution rate, leading to increased bioavailability.
- Our technology helps improve the stability of the drug, protecting it from degradation and enhancing the shelf-life of the medication.
Published Data
Technology: Solid dispersion Technology
Journal: International Journal of Pharmaceutics
IF: 5.8
Published: 2020
Results: This research aimed at developing oral thin films containing an antipsychotic drug-aripiprazole (ARP). As a BCS II class molecule, ARP requires enhancing its water solubility before formulating. Therefore, a solid dispersion of ARP – Poloxamer® 407 was prepared by ball milling and then incorporated into the films. The results show that co-processing led to an over 100-fold increase in drug solubility in comparison with pure drugs.
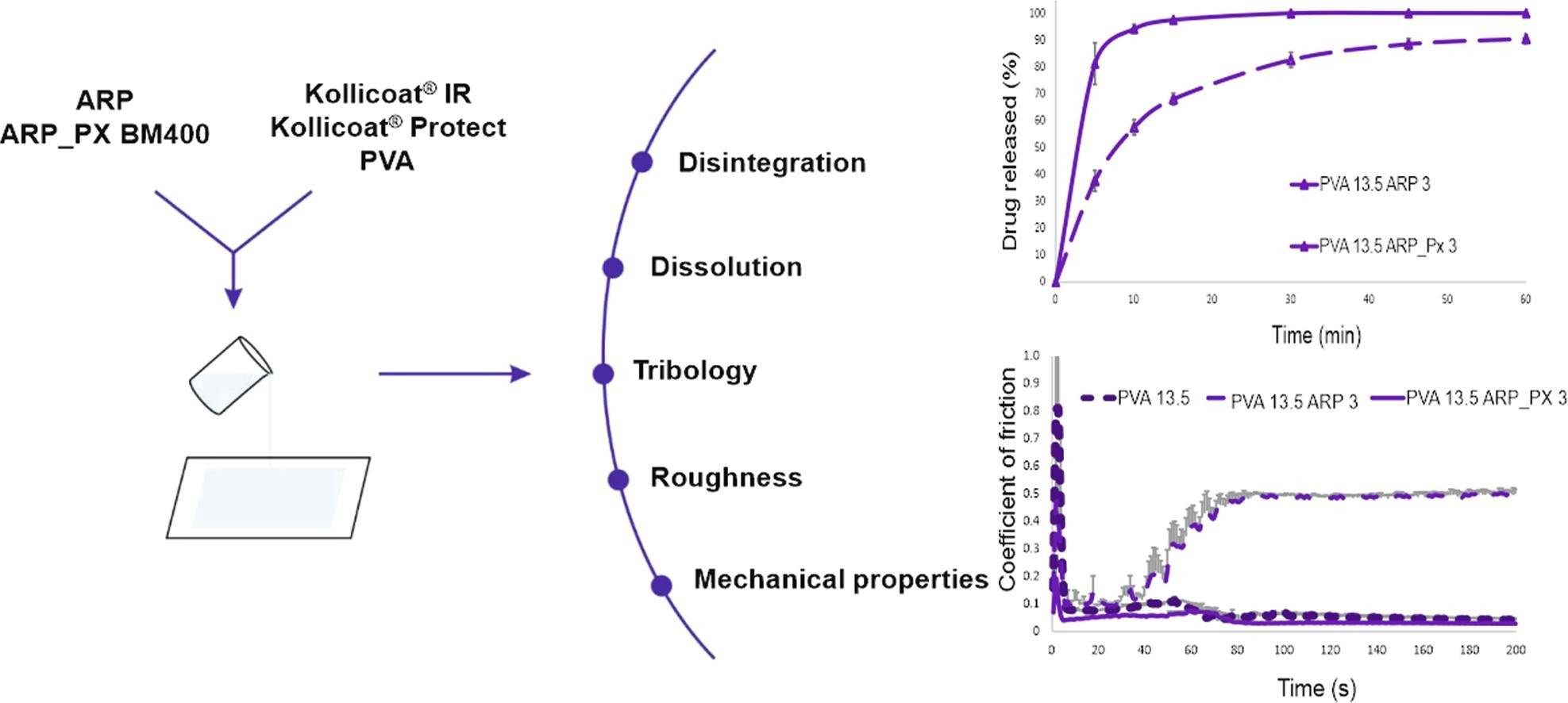 Fig.3 Advantages of solid dispersions. (Ewelina Łyszczarz, et al., 2020)
Fig.3 Advantages of solid dispersions. (Ewelina Łyszczarz, et al., 2020)
CD Formulation has extensive experience in solubilizing insoluble drugs and can provide solid dispersion solutions to meet customer needs. If you require our solid dispersion oral thin film preparation technology services, please contact us by phone or email, and our colleagues will get back to you within three working days.
References
- Randa Mohammed Zaki, Munerah Alfadhel, et al. Fabrication and characterization of orodispersible films loaded with solid dispersion to enhance Rosuvastatin calcium bioavailability. Saudi Pharmaceutical Journal. 2023, Vol (31):135-146.
- Ewelina Łyszczarz, Justyna Hofmanová, et al. Orodispersible films containing ball milled aripiprazole-poloxamer®407 solid dispersions. International Journal of Pharmaceutics. 2020, Vol (575).
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Solid dispersion preparation method. (Randa Mohammed Zaki, et al., 2023)
Fig.1 Solid dispersion preparation method. (Randa Mohammed Zaki, et al., 2023) Fig.2 Workflow of Oral Thin Film Solid Dispersion Preparation. (CD Formulation)
Fig.2 Workflow of Oral Thin Film Solid Dispersion Preparation. (CD Formulation) Fig.3 Advantages of solid dispersions. (Ewelina Łyszczarz, et al., 2020)
Fig.3 Advantages of solid dispersions. (Ewelina Łyszczarz, et al., 2020)
