Oral Nanoformulation Development
Inquiry
Oral nanoformulations have gained significant interest in medicine fields due to their unique preparation properties and potential applications. CD Formulation has been committed to oral nanoformulation research for many years, assisting researchers from various countries in the formulation development and commercial manufacturing of oral nanoformulations.
Advantages of Nanomertials in Oral Nanoformulations
Nanomaterial-based oral nanoformulations can improve drug stability in the harsh gastrointestinal (GI) tract environment, providing opportunities for targeting specific sites in the GI tract, increasing drug solubility and bioavailability, and providing sustained release in the GI tract. Its advantages include:
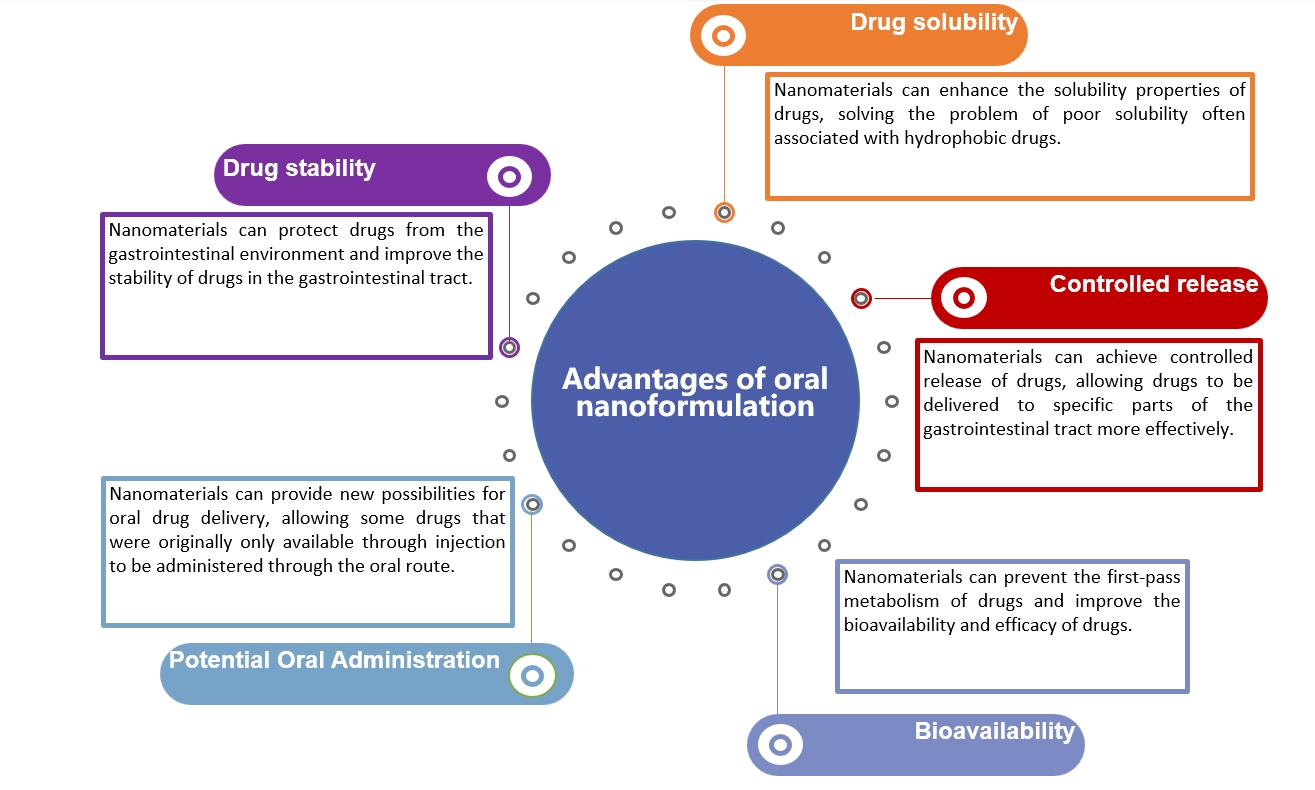 Fig.1 Advantages of nanomaterials in oral nanoformulation. (CD Formulation)
Fig.1 Advantages of nanomaterials in oral nanoformulation. (CD Formulation)
Our Oral Nanoformulation Development Services
The main bottleneck affecting the bioavailability of oral nanoformulation is biological barriers including gastric acid barrier and intestinal mucosal barrier. CD Formulation is committed to researching strategies based on organic and inorganic nanomaterials to solve this problem. It provides necessary technical support to global companies or researchers in improving the bioavailability of oral nanoformulation.
Our Strategies for Oral Nanoformulation Development
CD Formulation has advanced instruments, such as rapid nanomedicine preparation system, and rich experience in the oral nanoformulation development. We can provide organic nanomaterial or inorganic nanomaterial-based oral nanoformulation development service solutions based on our customers' existing research and development progress or new R&D requirements. Our research and development solutions include but are not limited to the following processes.
In-Depth Analysis of Drug Types and Metabolic Properties
At this stage, we conduct in-depth analysis of the physicochemical properties, pharmacokinetic properties (including absorption, distribution, metabolism, and elimination), drug targeting, and immunogenicity of your target small molecule drugs or biologic drugs.
Design of Carrier
Commonly used carrier structures include nanostructures, liposomes, hydrogels, membrane-controlled structures, and skeleton structures. We confirm the carrier type by analyzing their respective mechanisms of action and applicable drugs. It is an integral carrier (the drug will be dissolved or dispersed in the carrier matrix, and the drug will become an integral body with the delivery carrier through non-covalent bonds such as covalent bonds or electrostatic forces). Or it is a storage carrier (the drug is stored and delivered in the form of a package by the carrier, and the drug is carried to the target location and the release of the drug is controlled through the physical and chemical properties of the carrier or its modified group, such as active or passive targeting).
Nanocarrier Materials Screening and Preparation
We can screen and prepare nanocarrier materials including organic nanomaterials (polymeric nanoparticle, micelle, liposome, etc.) and inorganic nanomaterials (silica nanoparticle, porous nanoparticle, etc.).
Drug Loading Modification
Through the selection of carriers (nanomaterials), the design of drug loading methods, and the introduction of modification groups, we enable drugs to complete the circulation in the body conveniently and safely, achieve maximum utilization in the body, and achieve targeted delivery and improve transmission efficiency. and loading rate, protecting drugs, and reducing toxicity.
Targeting and Controlled Release
By decorating the drug surface with ligands that can bind to specific receptors on the disease surface, the drug can accurately reach diseased cells. At the same time, the carrier is monitored to release the active pharmaceutical ingredient at the appropriate time and location.
Evaluation and Testing
We also provide testing services such as analysis of physicochemical properties of nanomaterial components, characterization of oral nanoformulation, In vitro dissolution evaluation of oral nanoformulation, stabilization testing, and preclinical testing.
Scale-Up and Production of Oral Nanoformulation
Through research on the formulation process of oral nanoformulation, we confirm the feasibility of its scale-up and commercial production, and guide scale-up and industrial production according to customer needs.
Why Choose Us to Develop Oral Nanoformulations?
- Our core technical team has professional subject knowledge and skilled professional operation skills in the nanoformulation development, pilot scale-up production and clinical transformation, which is a professional guarantee for the smooth implementation of your project.
- We hold a variety of advanced instruments and equipment, such as rapid nanomedicine preparation system, which greatly saves raw materials and time in preparing samples during the formulation screening and optimization stages.
- We have accumulated rich experience in the formulation development of oral nanoformulations. We can provide customers with one-stop services for personalized professional research solutions, the design and preparation of nanomaterial carriers, formulation process development, quality control and scale-up production of oral nanoformulations.
Published Data
Technology: Based on insulin conjugated silver sulfide quantum dots and chitosan/glucose polymer nanotechnology
Journal: Nature Nanotechnology
IF: 31.713
Published: 2024
Results:
The authors developed a responsive oral insulin nanoformulation using a coating of insulin-conjugated silver sulfide quantum dots with chitosan/glucose polymers. The formulation is distributed to the liver of mice and rats after oral administration and promotes a dose-dependent reduction in blood glucose without causing hypoglycemia or weight gain in diabetic rodents. Therefore, this formulation shows the potential to control blood sugar orally without causing hypoglycemic episodes. The pictures of their study results are shown as follows.
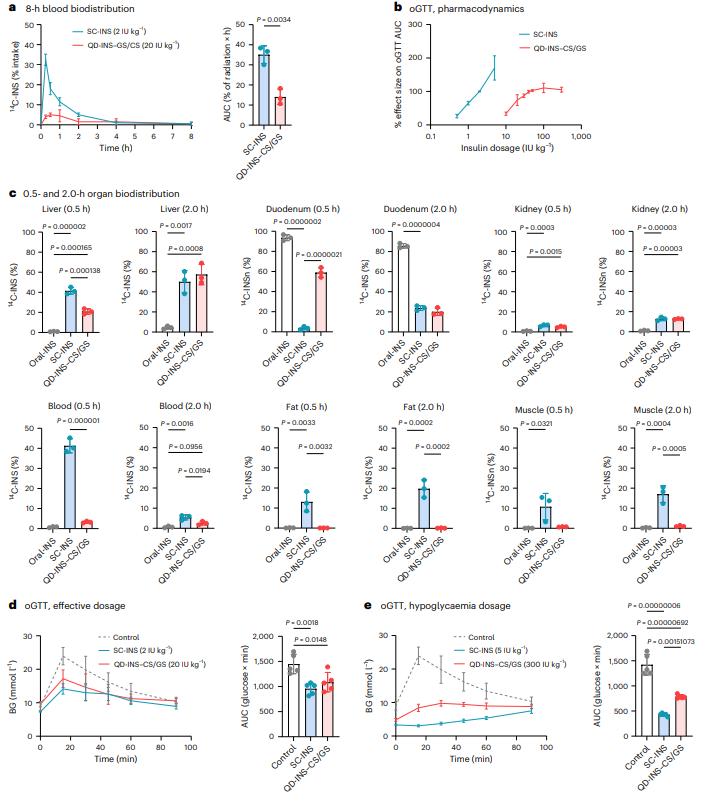 Fig.2 Pharmacokinetics and pharmacodynamics of a single dose of SC-INS, QD-INS–CS/GS and oral insulin alone. (Nicholas J. Hunt, et al., 2024)
Fig.2 Pharmacokinetics and pharmacodynamics of a single dose of SC-INS, QD-INS–CS/GS and oral insulin alone. (Nicholas J. Hunt, et al., 2024)
Oral nanoformulation has attracted close attention from many researchers because of its advantages such as ease of administration and good patient compliance. CD Formulation follows the golden trend in the research field and has achieved gratifying results in the nanoformulation process research and quality control research. If you are interested in our oral nanoformulation development services, please do not hesitate to contact us for more in-depth communication and discussion.
References
- Nicholas J. Hunt, Glen P. Lockwood, Scott J. Heffernan, et al. Oral nanotherapeutic formulation of insulin with reduced episodes of hypoglycaemia. Nature Nanotechnology. 2024, doi: 10.1038/s41565-023-01565-2.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Advantages of nanomaterials in oral nanoformulation. (CD Formulation)
Fig.1 Advantages of nanomaterials in oral nanoformulation. (CD Formulation)
 Fig.2 Pharmacokinetics and pharmacodynamics of a single dose of SC-INS, QD-INS–CS/GS and oral insulin alone. (Nicholas J. Hunt, et al., 2024)
Fig.2 Pharmacokinetics and pharmacodynamics of a single dose of SC-INS, QD-INS–CS/GS and oral insulin alone. (Nicholas J. Hunt, et al., 2024)
