Exosome Drug Delivery System for Nanoformulations
Inquiry
Exosomes are released by different cells in the body, each carrying unique biological components that enable them to transport drugs, nucleic acids, proteins, and other substances effectively. Exosomes offer several advantages, such as increased safety by avoiding immune detection, improved tolerance, longer circulation time, enhanced targeting capabilities through surface modifications, and the ability to easily penetrate cell membranes and biological barriers due to their small size and specific surface molecules. With the support of our advanced exosome technology platform, CD Formulation can establish exosome drug delivery systems to promote the clinical transformation and clinical applications of exosomes.
Advantages of Exosomes
Exosomes consist of a lipid bilayer made up of lipids surrounding a watery core. The lipid bilayer and the aqueous interior can hold hydrophobic or hydrophilic substances, respectively. Exosomes have various applications such as delivering DNA, RNA, and proteins, serving as diagnostic tools or imaging agents. Targeting ligands and covalent bonds can be added to the exosome surface. The advantages of exosomes as carriers of drug delivery systems are mainly as follows.
- Exosomes contain a large number of proteins and gene molecules, which have good compatibility and a huge intrinsic capacity for receiving cells.
- Cargo transported by exosomes can pass through membranes and avoid degradation, and these naturally produced vesicles have better tolerance in vivo.
- Exosomes as carriers of drug delivery systems have strong targeted delivery and homing capabilities and can be used for therapeutic purposes.
- Exosomes can be loaded with cargo in vivo by transfection and in vitro by electroporation and liposome transfection.
- Exosomes show weak nonspecific interactions with serum proteins.
Therefore, exosomes as carriers of drug delivery systems have good biocompatibility and can prolong the circulation half-life of therapeutic drugs.
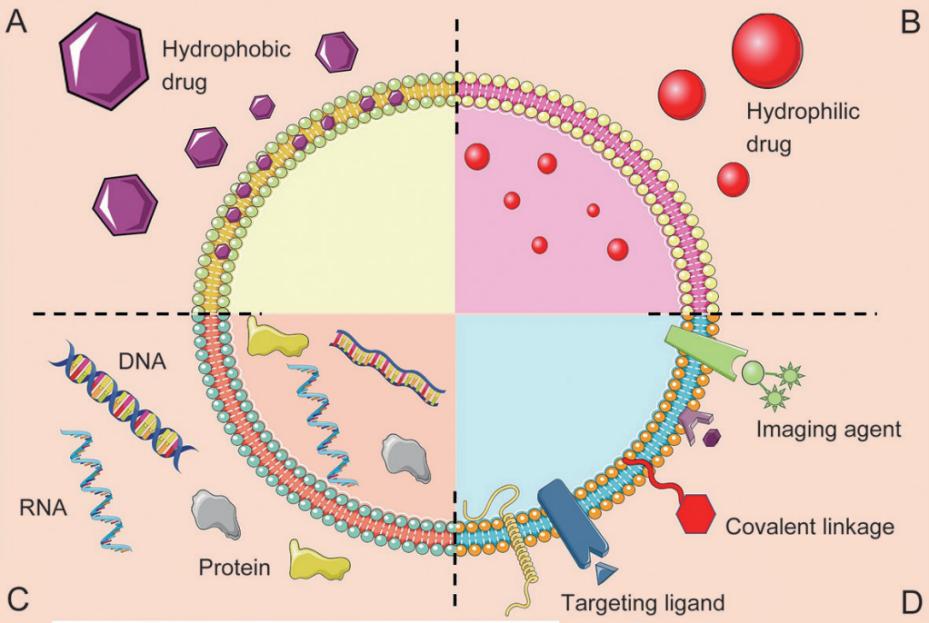 Fig.1 Schematic representation of the different types of exosome drug delivery systems. (Xin Luan, et al. 2017)
Fig.1 Schematic representation of the different types of exosome drug delivery systems. (Xin Luan, et al. 2017)
Clinical Applications of Exosome-based Drug Delivery Systems
Exosomes are considered an excellent natural carrier for transporting various substances and have significant potential in treating a wide range of diseases, such as cancers, inflammatory diseases, kidney diseases, cardiovascular diseases, etc.
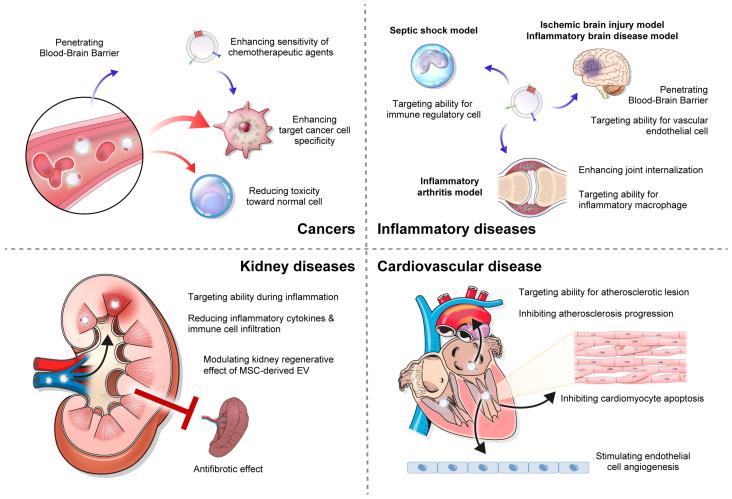 Fig.2 Clinical application of exosome-based drug delivery. (Hee Byung Koh, et al. 2023)
Fig.2 Clinical application of exosome-based drug delivery. (Hee Byung Koh, et al. 2023)
Our Methods for Exosome Drug Delivery System Development
CD Formulation relies on our advanced design concept and exosome technology platform to prepare exosomes, covering exosome isolation and purification, and drug loading.
Isolation and Purification
We typically use several methods for exosome isolation.
Sequential ultracentrifugation: We pellet the exosomes in sequential centrifugation steps based on their density.
Density gradient centrifugation: We further separate the exosomes based on their density in a dense medium.
Ultrafiltration: We use filters with specific pore sizes to concentrate the exosomes while eliminating larger particles.
Size exclusion chromatography: We use porous gel polymers as stationary phases and the starting biofluid as mobile phase to elute the particles, with larger vesicles eluting first and smaller vesicles later.
Immunoaffinity: We use specific antibodies that interact with specific membrane proteins of exosomes, which allows us to isolate exosomes of a specific source with high purity.
Precipitation: We use low-speed centrifugation to capture exosomes between 50-150 nm in a polymer mesh, and the addition of polymer components changes the solubility and partitioning properties of the exosomes, resulting in precipitation.
Drug Loading
There are two main techniques for loading drugs into exosomes, such as pre-loading (indirect loading) and post-loading (direct loading) methods. By selecting the appropriate loading method, we can customize the cargo content of exosomes to address specific therapeutic requirements using available technologies.
Pre-loading techniques involve loading the drug into donor cells before isolating exosomes, which results in the drug being encapsulated within the exosomes during their natural formation process. This can be achieved by modifying donor cells to produce targeted exosomes through co-incubation with the drug or transfection of the target gene. These modified cells are engineered to generate and release exosomes containing specific cargo like drugs, proteins, and nucleic acids.
Post-loading techniques entail loading the drug into pre-isolated natural exosomes. Within the post-loading approach, there are two distinct strategies: passive loading and active loading. Passive loading relies on the drug's physical properties to diffuse passively into the exosomes, while active loading involves employing techniques such as electroporation, sonication, and extrusion to encapsulate the drug into the exosomes.
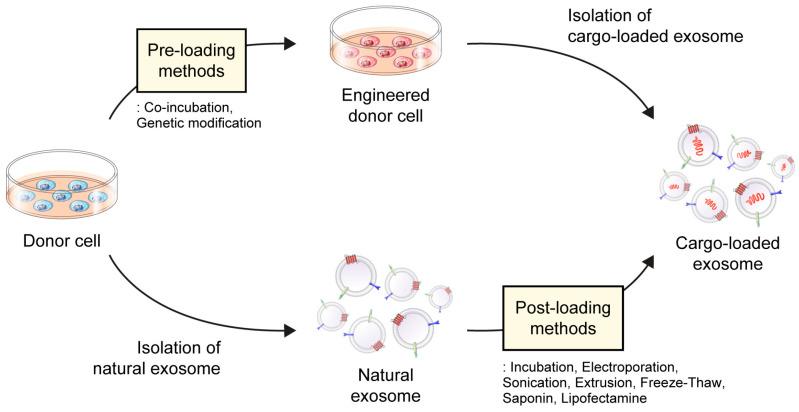 Fig.3 Drug-loading methods for exosomes. (Hee Byung Koh, et al. 2023)
Fig.3 Drug-loading methods for exosomes. (Hee Byung Koh, et al. 2023)
Highlights of Our Exosome Drug Delivery System Development
- At our advanced exosome technology platform, we have explored different drug-loading technologies to tailor the cargo content of exosomes to meet specific therapeutic needs.
- Our technical team modifies or supplements desired natural effects on the surface of exosomes through genetic and/or chemical engineering to enhance targeting and drug-loading capabilities.
- We are able to deliver various drugs via exosomes to achieve therapeutic purposes according to customer requirements, such as delivering small molecules via exosomes, delivering proteins via exosomes, and delivering genetic materials via exosomes.
Custom Exosome Development Services
We have conducted in-depth research on exosome development, and with our advanced exosome technology, we provide exosome formulation development and customization services. Our professional technical team has also committed to in-depth research on exosome drug delivery technology systems and can develop exosomes for different purposes to assist in the clinical transformation and application of exosomes.
Exosome Development for Nanomedicine
Relying on our advanced exosome technology, we can create specific exosomes according to customers' personalized requirements. After exploring and studying the unique properties, preparation technology and specific applications of exosomes, we can provide customers with high-quality exosome customization solutions to improve the purity and bioavailability of exosomes.
Published Data
Technology: Exosome-based drug delivery systems for glioma immunotherapy
Journal: Journal of Nanobiotechnology
IF: 10.2
Published: 2024
Results:
The authors discuss the preparation and application strategies of exosome-based glioma immunotherapy drug delivery systems, especially to enhance immunogenicity and reverse the immunosuppressive tumor microenvironment. Exosomes as drug carriers have the advantages of low toxicity, good biocompatibility, and intrinsic cell targeting, which can enhance the efficacy of glioma immunotherapy. In addition, the authors also analyze the challenges faced by exosome-based drug delivery systems in clinical translation. This exploration will guide the use of exosomes as drug carriers for glioma immunotherapy. Here are exosome-based immunotherapy strategies for glioma immunotherapy.
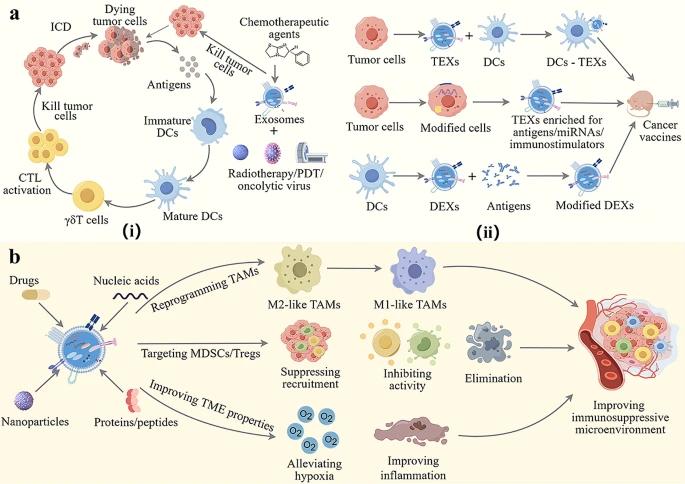 Fig.4 Exosome-based immunotherapy strategies for gliomas. (Xinqing Hao, et al. 2024)
Fig.4 Exosome-based immunotherapy strategies for gliomas. (Xinqing Hao, et al. 2024)
At CD Formulation, researchers have been actively exploring strategies to enhance the targeting capabilities of exosomes, such as using genetic modification technology to integrate specific biomolecules onto the surface or lumen of cells that produce exosomes. The surface of exosomes can be functionalized using ligands or peptides to enable them to recognize and bind to specific target cells or receptors. If you require our services, please contact us.
References
- Xin Luan, Kanokwan Sansanaphongpricha, Ila Myers, et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sinica. 2017, 1-10.
- Hee Byung Koh, Hyo Jeong Kim, Shin-Wook Kang, et al. Exosome-Based Drug Delivery: Translation from Bench to Clinic. Pharmaceutics. 2023, 15(8): 2042.
- Xinqing Hao, Shiming Wang, Liang Wang, et al. Exosomes as drug delivery systems in glioma immunotherapy. Journal of Nanobiotechnology. 2024, 22:340.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Schematic representation of the different types of exosome drug delivery systems. (Xin Luan, et al. 2017)
Fig.1 Schematic representation of the different types of exosome drug delivery systems. (Xin Luan, et al. 2017) Fig.2 Clinical application of exosome-based drug delivery. (Hee Byung Koh, et al. 2023)
Fig.2 Clinical application of exosome-based drug delivery. (Hee Byung Koh, et al. 2023) Fig.3 Drug-loading methods for exosomes. (Hee Byung Koh, et al. 2023)
Fig.3 Drug-loading methods for exosomes. (Hee Byung Koh, et al. 2023) Fig.4 Exosome-based immunotherapy strategies for gliomas. (Xinqing Hao, et al. 2024)
Fig.4 Exosome-based immunotherapy strategies for gliomas. (Xinqing Hao, et al. 2024)
