Biomembrane-based mRNA Delivery System for Biologics
Inquiry
Biomimetic membranes (such as cell membrane vesicles, bacterial-derived outer membrane vesicles, and extracellular vesicles) have enhanced mRNA drug delivery capabilities and immune evasion functions, thus providing a unique and promising platform for the effective and safe delivery of mRNA therapeutics. CD Formulation can help customers customize biomembrane materials for mRNA delivery, including cell membrane vesicles, bacterial-derived outer membrane vesicles, and extracellular vesicles.
Advantages of Biomembrane-based mRNA Delivery Systems
Biomembrane vectors are another novel biocompatible platform for mRNA delivery. Different types of biomembrane-based systems, including cell membrane vesicles, bacterial-derived outer membrane vesicles, and extracellular vesicles (e.g., exosomes), have been used for the delivery of therapeutic mRNA in vitro and in vivo.
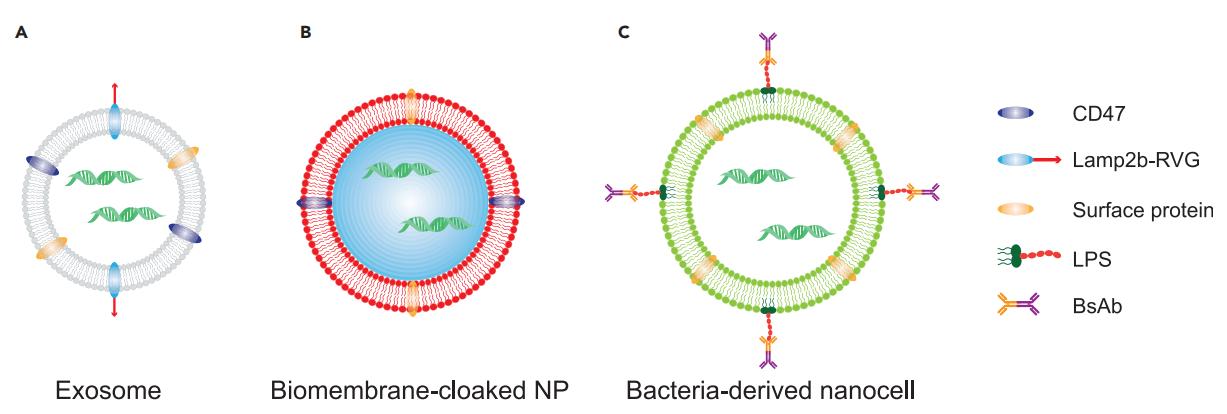 Fig.1 Biomembrane-Based Materials for RNA Delivery. (Xuexiang Han, et al. 2020)
Fig.1 Biomembrane-Based Materials for RNA Delivery. (Xuexiang Han, et al. 2020)
- Cell-based mRNA delivery systems have the advantages of higher biocompatibility, longer circulation period, endogenous intracellular and intercellular signals, etc.
- Extracellular vesicles (EVs) used for mRNA delivery include extracellular bilayer vesicles produced by multiple cell types. There are three main EV subtypes in mammals, including exosomes (50-150 nm), microvesicles (50-500 nm), and apoptotic bodies (>1 µm). Exosomes deliver mRNA through endocytosis and exocytosis. Microvesicles are produced by budding from the plasma membrane, and apoptotic bodies are characteristic of apoptosis.
- The use of cell membranes to wrap synthetic particles (such as gold, silica, lipid nanoparticles or polymers) forms a biomimetic mRNA delivery system. Cell membranes (such as red blood cells, platelet cells, immune cells, stem cells, tumor cells, or hybrid cells) can reduce the immunogenicity caused by synthetic particles while having the advantages of stability and tissue targeting.
Applications of Biomembrane-based mRNA Delivery Systems
Biomembrane-based mRNA delivery systems have shown great potential in various biomedical applications, including oncology, central nervous system diseases, obesity, and anti-inflammation.
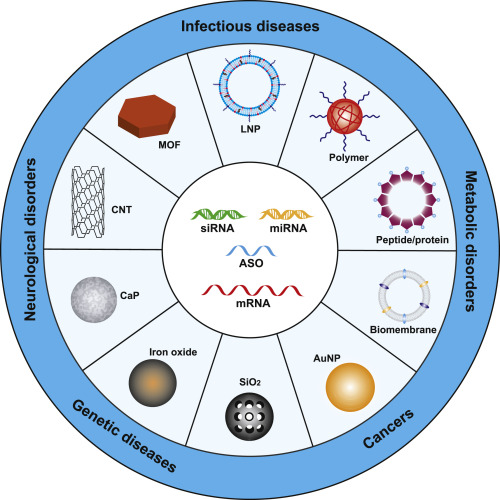 Fig.2 An Overview of Nanomaterial-Based RNA Delivery Platforms and Their Biomedical Applications. (Xuexiang Han, et al. 2020)
Fig.2 An Overview of Nanomaterial-Based RNA Delivery Platforms and Their Biomedical Applications. (Xuexiang Han, et al. 2020)
Our Solutions for Developing Biomembrane-based mRNA Delivery Systems
CD Formulation has established cell-based, extracellular vesicle-based, and bacterial-derived outer membrane vesicle-based mRNA delivery platforms by fusing biological (e.g., exosomes) and synthetic (e.g., nanoparticles) components to form hybrid particles. Such hybrid particles have the controllable manufacturing and stable storage capabilities of nanoparticles while retaining the biocompatibility and targeting specificity of biomembranes.
Cell Membrane-based mRNA Delivery Systems
Cell membrane-based delivery of mRNA therapeutics can be applied to general cell therapy. It uses the cell paracrine function to directly deliver proteins synthesized from mRNA introduced into cells in vitro. This type of cell-based delivery has biocompatibility, extended circulation life, and endogenous intracellular/intercellular signaling. Cells have been used as drug carriers to deliver enzymes, therapeutic drugs, or lipid particles to target sites. We can achieve extensive customization by introducing mRNA into a variety of available cell types (such as immune cells, blood cells, and mesenchymal cells) and through further genetic engineering.
Extracellular Vesicle-based mRNA Delivery Systems
We use extracellular vesicles as mRNA delivery vehicles. Extracellular vesicles comprise a heterogeneous group of extracellular double-membrane vesicles. Exosomes are the most studied and characterized extracellular vesicles, which shuttle and deliver their cargo between cells via endocytosis and exocytosis.
Bacterial-derived Outer Membrane Vesicle-based mRNA Delivery Systems
Outer membrane vesicles, as biological nanocarriers secreted by bacteria, contain rich bacterial components and can integrate adjuvants and carriers. We constructed an mRNA antigen delivery strategy in an outer membrane vesicle platform and successfully delivered mRNA into cells.
Our Workflow for Biomembrane-based mRNA Delivery System Development
Our development process for biomembrane-based mRNA delivery systems follows a structured workflow:
- Selection of Biomembrane Material: Based on the application, we select the appropriate biomembrane materials, such as cell membranes, outer membrane vesicles, or extracellular vesicles.
- Customization and Fabrication: Biomembrane materials are customized to encapsulate the desired mRNA payload, ensuring biocompatibility and targeting accuracy.
- Characterization and Analysis: The nanoparticles are characterized for size, surface charge, and stability to meet the project's specifications.
- Delivery Optimization: Our team works closely with clients to optimize the delivery system, ensuring effective mRNA transport and release.
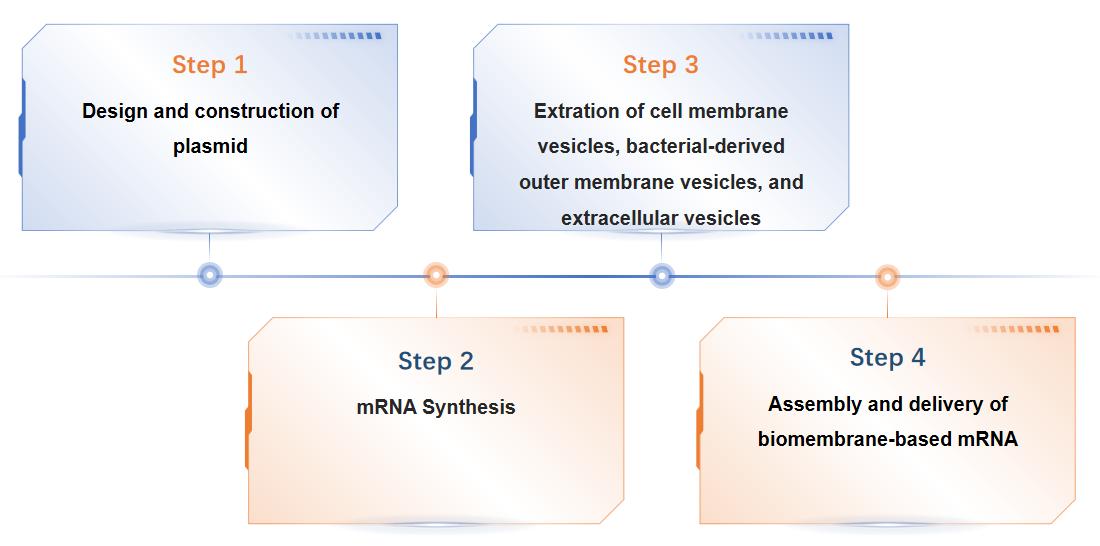 Fig.3 Our workflow for biomembrane-based mRNA delivery system. (CD Formulation)
Fig.3 Our workflow for biomembrane-based mRNA delivery system. (CD Formulation)
Why Choose CD Formulation?
- At our novel mRNA delivery system platform, we have established cell membrane vesicle-based, bacterial-derived outer membrane vesicle-based, and extracellular vesicle-based mRNA delivery systems.
- Our technical team has rich experience in exploring and researching the synthesis methods and extraction of biomembrane-based materials for mRNA delivery.
- We can provide clients with customizing biomembrane-based materials such as cell membrane vesicles, bacterial-derived outer membrane vesicles, and extracellular vesicles (e.g., exosomes).
Custom Exosome Development Services for mRNA Delivery
Biovesicles are less immunogenic and toxic, making them especially advantageous for repeated administration of mRNA. Biocompatible exosomes are emerging as a promising mRNA delivery platform. Leveraging our advanced mRNA delivery technology platform, CD Formulation specializes in developing customized exosome-based mRNA delivery systems tailored to meet each customer's unique needs.
Exosome Development for Nanomedicine
CD Formulation has lots of experience in customizing exosomes for mRNA delivery, covering mRNA design, isolation and purification of exosomes, mRNA binding, etc. We can also offer analysis and characterization of exosomes based on mRNA delivery to meet customers' different requirements.
Published Data
Technology: Genetically engineered cell membrane-coated nanoparticles for the cytosolic delivery of mRNA
Journal: Angewandte Chemie International Edition
IF: 16.1
Published: 2022
Results:
The authors describe cells that were genetically engineered to express the influenza virus fusion protein hemagglutinin. Membranes from the engineered cells were isolated and coated onto poly (lactic-co-glycolic acid) nanoparticle cores that were loaded with mRNA with the help of cationic lipid-like molecules. After the final influenza virus fusion protein hemagglutinin-mRNA-nanoparticle formulation was internalized by the target cell, the reduced pH in the late endosomes caused conformational changes in the influenza virus fusion protein hemagglutinin. The activated influenza virus fusion protein hemagglutinin then triggered membrane fusion, allowing the mRNA payload to escape into the cytosol to be translated into protein products. The results show that the virus-mimicking nanocarrier can deliver the mRNA payload to the cytosolic compartment after cellular uptake, thereby enhancing the expression of the encoded protein in vitro and in vivo.
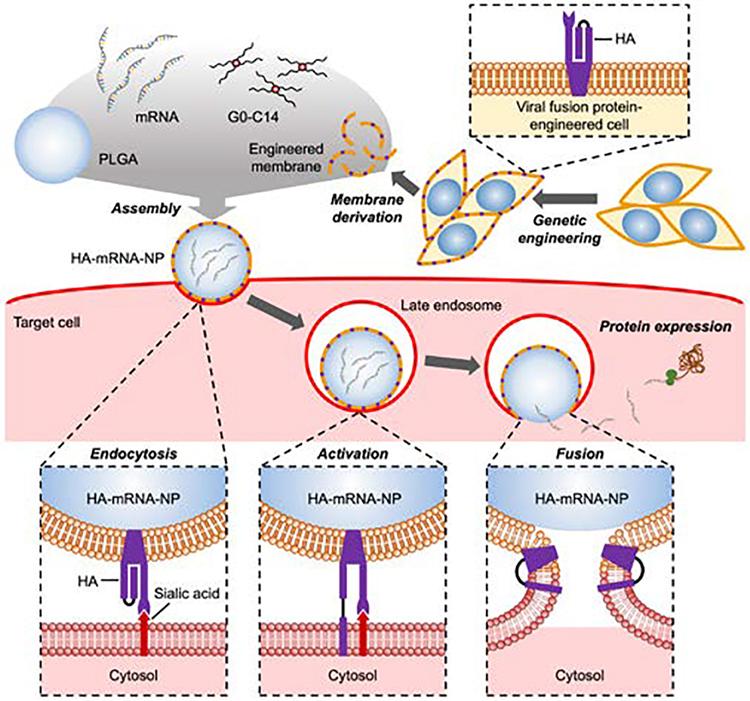 Fig.4 Schematic illustration of genetically engineered cell membrane-coated nanoparticles for the cytosolic delivery of mRNA. (Menghao Yin, et al. 2024)
Fig.4 Schematic illustration of genetically engineered cell membrane-coated nanoparticles for the cytosolic delivery of mRNA. (Menghao Yin, et al. 2024)
The mRNA-loaded biomembrane materials can deliver the mRNA to cells in a targeted manner, and at the same time, the double-membrane structure of the biomembrane material can protect the loaded mRNA from degradation by RNA enzymes in body fluids. Therefore, they can be used as ideal carriers for mRNA delivery. CD Formulation has established a biomembrane-based mRNA delivery platform to enhance mRNA delivery efficiency. If you are interested in biomembrane-based mRNA delivery systems for biologics, please contact us for detailed communication.
References
- Xuexiang Han, Michael J. Mitchell, Guangjun Nie. Nanomaterials for Therapeutic RNA Delivery. Matter. 2020,3(6):1948-1975.
- Joon Ho Park, Animesh Mohapatra, Jiarong Zhou, et al. Virus-Mimicking Cell Membrane-Coated Nanoparticles for Cytosolic Delivery of mRNA. Angewandte Chemie International Edition. 2022,61(2): e202113671.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Biomembrane-Based Materials for RNA Delivery. (Xuexiang Han, et al. 2020)
Fig.1 Biomembrane-Based Materials for RNA Delivery. (Xuexiang Han, et al. 2020) Fig.2 An Overview of Nanomaterial-Based RNA Delivery Platforms and Their Biomedical Applications. (Xuexiang Han, et al. 2020)
Fig.2 An Overview of Nanomaterial-Based RNA Delivery Platforms and Their Biomedical Applications. (Xuexiang Han, et al. 2020) Fig.3 Our workflow for biomembrane-based mRNA delivery system. (CD Formulation)
Fig.3 Our workflow for biomembrane-based mRNA delivery system. (CD Formulation) Fig.4 Schematic illustration of genetically engineered cell membrane-coated nanoparticles for the cytosolic delivery of mRNA. (Menghao Yin, et al. 2024)
Fig.4 Schematic illustration of genetically engineered cell membrane-coated nanoparticles for the cytosolic delivery of mRNA. (Menghao Yin, et al. 2024)
