Albumin Nanoparticle Technology for Nanoformulations
Inquiry
Albumin nanoparticles have many advantages that can promote their clinical application, and many of our studies have explored various albumin nanoparticles and their applications in various fields such as drug delivery, bioimaging, and theranostics. CD Formulation can provide our clients with albumin nanoparticles technology customization services for albumin nanoparticles to meet the different requirements depending on our advanced albumin nanoparticle technology platform.
Advantages of Albumin Nanoparticles
Albumin-based nanoparticles exhibit unique capabilities, including the successful management of drug-resistant and orthotopic brain tumors. These nanoparticles not only facilitate targeted drug delivery to tumors but also mitigate drug-induced toxicity in living organisms. Moreover, the attachment of various targeting ligands (e.g., nanobodies, fatty acids, peptides, glycyrrhetinic acid, antibodies, mannose, and TNF-related apoptosis-inducing ligands) to albumin-based nanoparticles can enhance the selective uptake of nanoparticles by cancer cells.
Applications of Albumin Nanoparticle Technology
The advancement of utilizing albumin-based nanoparticles in drug delivery and bioimaging plays a crucial role in enhancing the biocompatibility of these nanoparticles, thereby facilitating their potential clinical application. These nanoparticles can be loaded with various active substances such as chemotherapeutic drugs, pulmonary medications, inhibitors, dyes, contrast agents, or a combination of these agents. The formulated albumin-based nanoparticles have versatile applications including chemotherapy, photothermal therapy (PTT), photodynamic therapy (PDT), combined therapy, optical imaging, magnetic resonance imaging (MRI), photoacoustic tomography, multimodal imaging, and therapeutic diagnosis.
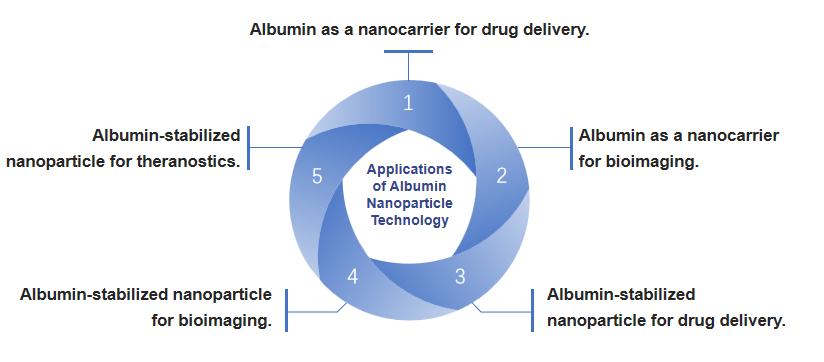 Fig.1 Applications of albumin nanoparticle technology. (CD Formulation)
Fig.1 Applications of albumin nanoparticle technology. (CD Formulation)
Albumin Nanoparticle Technology at CD Formulation
CD Formulation relies on our advanced design concept and our mature albumin nanoparticle technology platform to prepare and characterize albumin nanoparticles to meet customers' requirements. At our albumin nanoparticle technology platform, we have constructed the following preparation methods (such as desolvation, emulsification, nanoparticle albumin bond technology, etc.) for albumin nanoparticle development.
Albumin Nanoparticle Production Technologies
Our albumin nanoparticle production technologies are divided into chemical methods and physical methods. Chemical methods (such as desolvation, emulsification and self-assembly) use chemical additives such as ethanol, cottonseed oil or β-mercaptoethanol to induce the formation of nanoparticles, while physical methods (such as nanospray drying, thermal gelation and nanoassembly technology) use physical factors such as heat or pressure to generate nanoparticles.
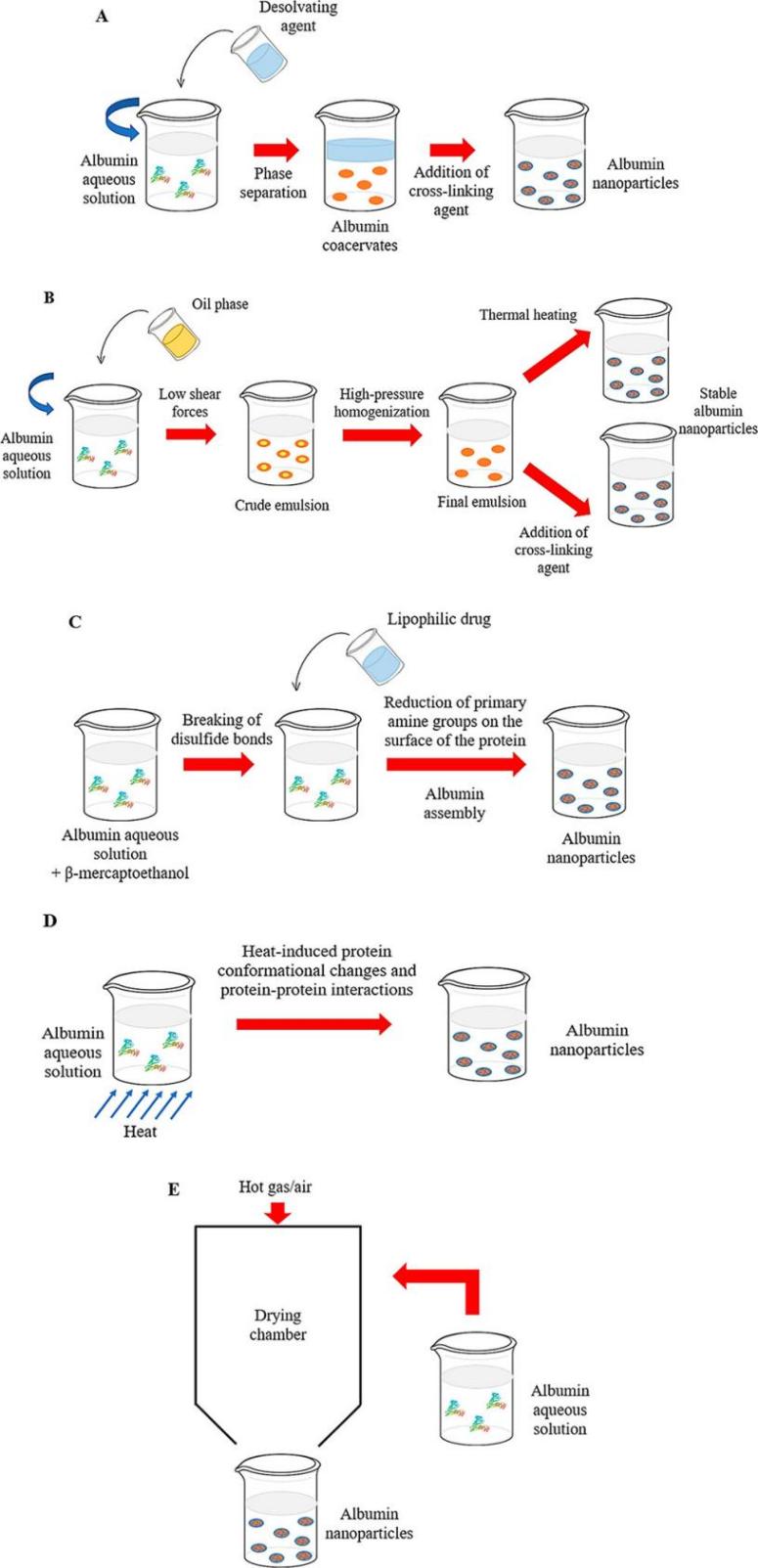 Fig.2 Main methods for the preparation of albumin nanoparticles: (A) desolvation, (B) emulsification, (C) self-assembly, (D) thermal gelation, (E) nanospray drying. (Alessandra Spada, et al. 2021)
Fig.2 Main methods for the preparation of albumin nanoparticles: (A) desolvation, (B) emulsification, (C) self-assembly, (D) thermal gelation, (E) nanospray drying. (Alessandra Spada, et al. 2021)
- Desolvation (Coacervation): This method is robust, reproducible, has the absence of toxic organic solvents, simplicity, and the possibility to obtain smaller-sized nanoparticles.
- Emulsification: We can use this method to gain higher drug encapsulation efficiency.
- Self-assembly: This method is always widely used for high loading of poorly water-soluble drugs.
- Thermal gelation: This method is mainly used for possible fabrication of nanoscale hydrogels.
- Nanospray drying: This method only needs a single-step continuous and scalable process and is a versatile technique and useful for heat-sensitive samples, and control for particle size.
- Microfluidic mixing: This method has the following advantages such as tunable size, structure, and surface, narrow size distribution, controlled release profile, high versatility and reproducibility, smaller size nanoparticle, low reagent consumption, better mixing, better drug loading capability.
- Nanoparticle Albumin Bound (NAB) Technology: Our NAB technology is a nanotechnology-based drug delivery system that exploits the intrinsic properties of albumin to achieve selective and efficient delivery of hydrophobic drugs without the use of toxic solvents. This method is mainly used for encapsulating lipophilic drugs, is safe and suitable for intravenous usage of poorly soluble drugs, has no requirements for surfactants or polymeric materials for preparation, disulfide formation induced by homogenization does not substantially denature HAS, has higher drug content, smaller size.
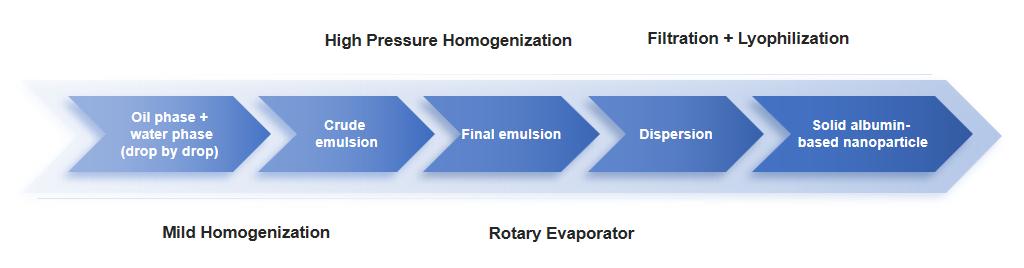 Fig.3 Nanoparticle albumin-bound technology possess. (CD Formulation)
Fig.3 Nanoparticle albumin-bound technology possess. (CD Formulation)
Albumin Nanoparticle Characterization Technologies
CD Formulation has lots of experience in characterizing albumin nanoparticles with the help of our albumin nanoparticle technology platform. We hold a variety of leading and precise instruments and equipment (such as SET, TEM, PLM, XRD, X-ray scattering, ELS, etc.) for the characterization of albumin nanoparticles.
Highlights of Our Albumin Nanoparticle Technology Platform
- We have established an albumin nanoparticle technology platform and can provide customers with our high-quality albumin nanoparticle fabrication and characterization of albumin nanoparticles to meet customers' different needs.
- We hold advanced instruments and equipment for the fabrication and characterization of albumin nanoparticles, including high-pressure homogenizer, spray dryer, SET, TEM, PLM, XRD, X-ray scattering, ELS, etc.
- Our team has rich experience in exploring and constructing albumin nanoparticles and can provide a quick response to customers' requirements for albumin nanoparticle formulation development and characterization immediately.
Custom Albumin Nanoparticle Development Services
Utilizing our albumin nanoparticle technology, we provide personalized development services for albumin nanoparticles that are designed to meet the individual requirements of our customers. We have a thorough knowledge of the distinct characteristics of albumin nanoparticles and their uses, and we are dedicated to creating tailored solutions that improve the effectiveness and durability of different pharmaceutical and biotechnological items.
Albumin Nanoparticle Development for Nanomedicine
CD Formulation excels in developing albumin nanoparticles for nanomedicine, utilizing advanced albumin nanoparticle technology. Our skilled team develops these nanoparticles for diverse applications and deeply explores their clinical use, fostering their medical and market viability.
Published Data
Technology: Nanoparticle albumin bound (nab™) technology
Journal: European Journal of Pharmaceutics and Biopharmaceutics
IF: 4.9
Published: 2023
Results:
The authors compared the marketed product Abraxane
® with albumin-stabilized nanosuspensions of the poorly water-soluble drug itraconazole with the goal of determining the critical product parameters of the nanosuspension in terms of the manufacturing process to evaluate the transferability of the nab™ technology to other drugs. In parallel, the authors analyzed the colloidal properties, stabilizing protein composition, and particle disintegration behavior. The authors also investigated the impact of the critical process step, high-pressure homogenization, using a design of experiments (DoE) approach. A nanosuspension containing spherical, stable drug nanoparticles stabilized by a large portion of dissolved albumin surrounding the nanoparticles was identified. The results demonstrate the transferability of the nab™ technology to select other poorly water-soluble drugs with the significant advantage of eliminating solubilizers and emulsifiers for intravenous applications.
CD Formulation can provide global researchers with albumin nanoparticle technology services depending on our advanced albumin nanoparticle technology platform and strong nanodrug design capabilities. If you need any questions about albumin nanoparticle technology services, please do feel free to contact us.
References
- Alessandra Spada, Jaber Emami, Jack A Tuszynski, et al. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Molecular Pharmaceutics. 2021,18(5):1862-1894.
- Annika Adick, Werner Hoheisel, Stefan Schneid, et al. Challenges of nanoparticle albumin bound (nab™) technology: Comparative study of Abraxane® with a newly developed albumin-stabilized itraconazole nanosuspension. 2023,193:129-143.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Applications of albumin nanoparticle technology. (CD Formulation)
Fig.1 Applications of albumin nanoparticle technology. (CD Formulation) Fig.2 Main methods for the preparation of albumin nanoparticles: (A) desolvation, (B) emulsification, (C) self-assembly, (D) thermal gelation, (E) nanospray drying. (Alessandra Spada, et al. 2021)
Fig.2 Main methods for the preparation of albumin nanoparticles: (A) desolvation, (B) emulsification, (C) self-assembly, (D) thermal gelation, (E) nanospray drying. (Alessandra Spada, et al. 2021) Fig.3 Nanoparticle albumin-bound technology possess. (CD Formulation)
Fig.3 Nanoparticle albumin-bound technology possess. (CD Formulation)
