Gene Therapy Formulation Genotoxicity Assessment
Inquiry
Determination of genotoxicity as an important determination of toxicity assay for evaluating gene therapy formulations. Genotoxicity assessment of gene therapy formulations is the core link to ensure the safety and efficacy of gene therapy formulations. In-depth investigation and accurate quantification of genotoxicity have also become an indispensable part of evaluating the reliability and efficacy of gene therapy formulation development. Genotoxicity measurement of gene therapy formulations can effectively and accurately predict the risks that may arise after administration, and accordingly optimize the formulation design to minimize adverse effects. To guarantee the safety and effectiveness of developing gene therapy formulations and to expedite the process, CD Formulation offers precise and trustworthy genotoxicity assessments of gene therapy formulations upon request.
The Importance of Gene Therapy Formulation Genotoxicity Assessment
The development of gene therapy formulations involves the introduction of exogenous genes, which may trigger genomic instability, especially integrated gene therapy vectors, which can insert or modify the genome of the target cells. For example, like various viral vectors including lentiviruses, retroviruses, etc, random insertion into the host's genome may lead to the emergence of genotoxicity, cellular cancer and other risks, so for example, to ensure the safety of gene therapy formulations for the host, especially some of the viral vectors based on the gene therapy agent, we need to carry out a rigorous genotoxicity test, which not only ensures the safety and achieve the desired effect of the application of gene therapy formulations, but also ensures that the researchers developed gene therapy formulations fully comply with the regulatory needs of the relevant departments.
Explore Our Gene Therapy Formulation Genotoxicity Assessment
Mutagenicity assessment
Mutagenicity assessment of gene therapy formulations is used to assess whether a gene therapy formulation is at risk of mutation, primarily through in vitro and in vivo testing, by examining the potential for gene therapy vectors, to induce mutations in different biological models, or different cells. Mutagenicity testing of the developed gene therapy formulation is important to ensure that the developed biopharmaceutical formulation meets the relevant genotoxicity requirements. Commonly used mutability testing methods include the Ames test, mammalian cell gene mutation assay, and others.
Chromosome damage assessment
The assessment of chromosomal damage is intended to screen for gene therapy agents that induce structural or numerical changes in chromosomes in cells, including, but not limited to, chromosomal abnormalities and karyorrhexis disorders. Chromosome damage may cause a variety of problems, such as leading to host cell apoptosis and the onset of dysfunction. We offer chromosomal damage testing including, but not limited to, mammalian micronucleus analysis and chromosomal aberration analysis techniques. Relying on our well-established and advanced technology platform, we can help researchers evaluate chromosomal chromosome loss assessment of gene therapy formulations from a multi-dimensional perspective to further ensure the safety of gene therapy formulation development.
Insertion mutagenicity assessment
In the process of gene therapy development, commonly used viral vectors have random insertion positions when importing exogenous sequences, and this randomness may cause the inactivation of normal genes and thus produce more negative effects. Therefore, it is particularly important to detect the integration sites of recombinant cells. Insertion mutagenicity assessment serves as an integral part of the development process of gene therapy formulations. We can accurately assess the integration sites of recombinant cells and their potential risks through whole genome sequencing, LM-PCR combined with second-generation sequencing and other advanced technologies, providing a strong guarantee for the safety and efficacy of gene therapy formulations.
Long-term genomic stability assessment
Gene therapy agents may be present in host cells for long periods and therefore need to be assessed for their long-term effects on the host genome. Such assessments include long-term in vivo and in vitro experiments to observe whether irreversible changes have occurred in the genome. This assessment aspect can help determine the long-term safety of gene therapy formulations, especially in the case of integrated gene vectors.
 Fig.1 Our process of gene therapy formulation genotoxicity assessment. (CD Formulation)
Fig.1 Our process of gene therapy formulation genotoxicity assessment. (CD Formulation)
Our Test Method of Gene Therapy Formulation Genotoxicity Assessment
Ames test
Using bacteria as test subjects to observe the mutation of bacterial genes by the subjects.
Chromosome aberration test
Characteristic animal cells are usually used as test cells. The test cells are exposed to the test material under in vitro culture conditions, and then the structural and quantitative changes of the chromosomes are evaluated by staining of the cellular chromosomes and microscopic observation.
Micronucleus test
It is a commonly used genotoxicity test method for assessing the extent of genetic damage to biological cells caused by a test substance. The test determines whether a substance is genotoxic mainly by observing the formation of micronuclei in the cell nucleus.
Integration site analysis
Integration of viral DNA into the host chromosome may be an early event in the oncogenic process. Therefore, regulatory authorities require integration site analysis (ISA) for all potentially integrating viruses. ISA is usually performed on DNA extracted from animal tissue samples at least several weeks after a single dose.
Our Instruments for Gene Therapy Formulation Genotoxicity Assessment
| Different Tests |
Content Description |
| Ames' existing instruments |
Clean bench, biosafety cabinet, oven, incubator, refrigerator, freezer, ultra-low temperature refrigerator, autoclave, water purifier, balance, pipette, enzyme marker, gas bath shaker, vortex mixer, dispenser pump, miniature benchtop vacuum pump. |
| Chromosome test existing instruments |
Centrifuge, electric thermostatic water bath, refrigerator, freezer, ultra-low temperature refrigerator, autoclave, water purifier, water sandwich carbon dioxide incubator, automatic cell counter, inverted microscope, pipette, microscope, clean bench. |
| Available instruments for micronucleus test |
Microscope |
Highlights of Our Gene Therapy Formulation Genotoxicity Assessment
- We have a state-of-the-art platform with experienced scientists to ensure that the safety assessment of gene therapy formulations is conducted in the most professional manner while complying with global regulatory standards.
- We have an experienced team of experts who can provide invaluable support and guidance to our clients throughout the development lifecycle.
- We can provide flexible service options according to our client's needs to help them effectively meet their development goals.
- We have a specialized team and hands-on experience in the safety evaluation of gene therapy agents to ensure high-quality data and fast turnaround times.
Published Data
Technology: Genotoxicity assessment
Journal: Mol Ther Methods Clin Dev
IF: 4.7
Published: 2017
Retroviral vectors have entered the clinical arena and have shown promising results in the treatment of immunodeficiency, malnutrition and other diseases. However, gene therapy still leads to some adverse events, which have been found to be associated with the integration of semi-random vectors into the host cell genome resulting in dysregulation of neighboring proto-oncogenes. Although improvements in vector design have significantly reduced the risk of such insertion mutagenesis, the analysis of potential genotoxicity and consequences of vector integration remains an important parameter for basic and translational research, and most importantly for clinical purposes. This article focuses on current assays for analyzing biodistribution and genotoxicity in the preclinical setting.
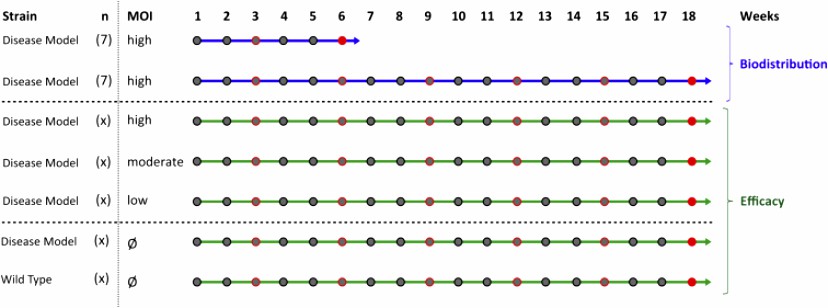 Fig.2 Pre-clinical pharmacodynamics and biodistribution for hematopoietic gene therapy. (Biasco L,et al., 2017)
Fig.2 Pre-clinical pharmacodynamics and biodistribution for hematopoietic gene therapy. (Biasco L,et al., 2017)
Genotoxicity testing is an important tool for assessing the genetic safety of gene therapy formulations, and it provides key information about the effects and potential risks of gene therapy products on genetic material. CD Formulation provides genotoxicity testing for gene therapy formulations, which can help researchers to be able to better understand the genotoxicity of gene therapy formulations, and provide key information for the subsequent clinical application and long-term use of the drug. If you are interested in us, please feel free to contact us.
Reference
- Biasco L, et al. Analyzing the Genotoxicity of Retroviral Vectors in Hematopoietic Cell Gene Therapy. Mol Ther Methods Clin Dev. 2017, 8:21-30.
Related Services

 Fig.1 Our process of gene therapy formulation genotoxicity assessment. (CD Formulation)
Fig.1 Our process of gene therapy formulation genotoxicity assessment. (CD Formulation)  Fig.2 Pre-clinical pharmacodynamics and biodistribution for hematopoietic gene therapy. (Biasco L,et al., 2017)
Fig.2 Pre-clinical pharmacodynamics and biodistribution for hematopoietic gene therapy. (Biasco L,et al., 2017)