Gene Therapy Formulation Screening Service
Inquiry
During the gene formulation development phase, we need to screen formulations for their ultimate route of administration and clinical application to ensure that the target gene therapy formulation is ultimately available. CD Formulation has been an industry leader in the development of gene therapy formulations, and based on our advanced drug formulation development platforms and experienced team of specialists, we aim to maximize your ability to advance the screening and development of gene therapy formulations. We aim to maximize your ability to advance the screening and development of gene therapy formulations based on our advanced drug formulation development platform and experienced team of experts. Through precise experiments and tests, we will ensure that the designed formulations have good performance, including safety, stability, efficacy and so on.
Why Conduct Gene Therapy Formulation Early Screening?
- Reduce R&D costs. Early screening helps to eliminate ineffective or inefficient formulations in the early stages of drug development, reducing the cost of investment in subsequent studies.
- Reduce immune response and improve safety. Early screening allows for the evaluation and optimization of gene therapy vectors to reduce immune responses in patients and improve the safety of the treatment.
- Identify and validate targets. Early screening can be used to identify genes or biomarkers associated with specific diseases, providing accurate targets for subsequent therapeutic strategies.
- Assess efficacy and side effects. Early screening can be used to assess the efficacy and possible side effects of potential therapeutic regimens at the preclinical stage, ensuring that candidates entering clinical trials have a reasonable risk-benefit ratio.
- Improve trial success. Early screening can improve the success rate of gene therapy by ensuring the effectiveness of therapeutic strategies before they enter the more expensive and complex phase of clinical trials.
Explore Our Gene Therapy Formulation Screening
Based on our years of research and discovery in gene therapy formulation development, we provide outstanding technical services in early screening studies of gene therapy formulations. In this phase, we optimize and conduct early screening of a wide range of formulation conditions, including route of administration, excipient selection, and physicochemical properties, based on client project requirements and experimental objectives. For example, in excipient selection, we prioritize excipients that can enhance stability, improve delivery efficiency, or reduce immune response, and make sure to ensure the compatibility of all excipients with the active ingredient and other excipients, and so on. When target formulations are identified, we test all aspects of the gene therapy product, including stability, efficacy and feasibility, to ensure that the formulation is consistent with your product. Our experts in genetic formulation development are committed to providing the highest quality formulation early screening and development services. We list some of them here.
- Lyophilized formulations. This form of formulation allows the gene therapy product to remain stable after the removal of water and is usually active for a longer period at room temperature. This is particularly important when long-term storage is required or for use in areas lacking cold chain facilities.
- Liquid formulations. Some gene therapy products are available in liquid form and often require refrigeration or freezing to maintain their stability and effectiveness.
- Spray-dried formulations. Solid powders are prepared by spray-drying techniques that can be used for inhalation or mucosal administration.
- Liposomal formulations. Utilization of liposomes as carriers can enhance cellular uptake of gene therapy drugs and protect therapeutic genes from enzymatic degradation in the body.
- Nanoparticle formulations. Formulations prepared by nanotechnology can improve drug delivery efficiency and tissue targeting.
What Solutions Can We Offer for Gene Therapy Formulation Screening?
- Evaluation of efficiency. Evaluate the delivery efficiency of different formulations, including transduction efficiency and gene expression levels.
- Immunogenicity assessment. Evaluate the immune response that may be induced by gene therapy formulations and screen for less immunogenic formulations to improve the safety of the treatment.
- Safety assessment. Evaluate the safety of gene therapy formulations in vitro and animal models, including potential off-target effects, insertional mutagenesis, and long-term toxicity.
- Pharmacodynamic studies. Evaluate the efficacy of gene therapy formulations in cellular and animal models, including the degree of improvement in disease models.
- Stability studies. Screening for conditions that maintain the stability of gene therapy formulations, including storage and transportation conditions, as well as formulation forms suitable for clinical use.
The screening of gene therapy agents can help to reduce the problems faced by gene therapy agents in development by performing an early analysis from multiple dimensions such as safety, stability, efficacy, and immunogenicity.
 Fig.1 Gene therapy formulation screening. (CD Formulation)
Fig.1 Gene therapy formulation screening. (CD Formulation)
Our Technologies for Gene Therapy Formulation Screening
| Technologies |
Content Description |
| Freeze-drying (lyophilization) technology platforms |
For the development of stabilized lyophilized formulations to improve the long-term stability and convenience of formulations. |
| Surfactant and cryoprotectant screening |
For improving the physical stability of gene therapy formulations and protection during the freeze-drying process. |
| Viral vector production and purification technology platform |
We have established a comprehensive viral vector production and purification technology platform, including but not limited to the provision of comprehensive technical services including virus packaging, concentration determination, purification and titer measurement. |
Highlights of Our Gene Therapy Formulation Screening
- We have extensive expertise in developing a wide range of gene therapy formulation dosage forms and gene delivery pathways.
- We provide full process technical services for gene formulation development, including but not limited to in vitro cell culture studies, in vivo potency assays, etc.
- We can help our customers obtain optimal gene therapy formulations by continuously optimizing the formulation system during formulation screening.
- We have advanced gene therapy formulation development platforms, including but not limited to liposome technology, nanoparticle technology, and various gene delivery systems.
- We provide full process services from formulation screening, design, and process development to the production of formulations.
Published Data
Technology: Developing gene therapy formulations
Journal: Respir Care
Published: 2015
This study describes how the success of these novel aerosol applications will depend on a number of factors, such as the development of gene therapy formulations that can be safely delivered acutely and chronically to the lungs, improved delivery of genetic material beyond the mucus barrier of the airways and cell membranes with transfer of the material into the cytoplasm or nucleus, the development of an aerosol device that can efficiently deliver genetic material and peptides to a lung target in a short period of time, the development of a device that can increase delivery of peptides to infants' aerosol delivery to the lungs, development of devices that increase aerosol delivery to infants, optimization of bioavailability of systemically delivered peptides, etc.
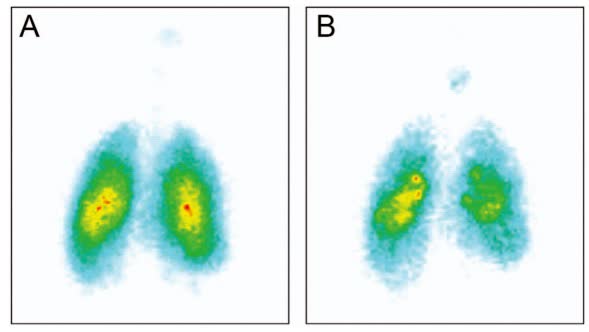 Fig.2 Ventilation and drug deposition scans with alpha-1 antitrypsin deficiency. (Laube BL, et al., 2015)
Fig.2 Ventilation and drug deposition scans with alpha-1 antitrypsin deficiency. (Laube BL, et al., 2015)
CD Formulation is an industry leader in the screening of gene therapy formulations, and based on our advanced technology platform, we aim to provide comprehensive solutions for the development of gene therapy formulations. If you are interested in us, please feel free to contact us.
References
- Laube BL, et al. Aerosolized Medications for Gene and Peptide Therapy. Respir Care. 2015, 60(6):806-21.
Related Services

 Fig.1 Gene therapy formulation screening. (CD Formulation)
Fig.1 Gene therapy formulation screening. (CD Formulation) Fig.2 Ventilation and drug deposition scans with alpha-1 antitrypsin deficiency. (Laube BL, et al., 2015)
Fig.2 Ventilation and drug deposition scans with alpha-1 antitrypsin deficiency. (Laube BL, et al., 2015)