Lyophilized Gene Therapy Formulation Development
Inquiry
Gene therapy formulations rely on low-temperature transportation, which not only increases transportation and logistics costs but also affects the stability of the formulations. The above problems can be solved by developing a lyophilization process for gene therapy formulations. CD Formulation provides comprehensive technical support for gene therapy lyophilization formulations, and we have brought breakthroughs in the storage of gene therapy formulations through the research of lyophilization protective agent components, screening and optimization, and the stability of lyophilized products. For example, we have developed lentiviral vector lyophilized preparations, which can achieve the advantages of long-time storage, easy transportation and improved stability.
Advantages of Lyophilized Gene Therapy Formulation
- Long-term stability. Lyophilization technology removes water from drug formulations, thereby reducing microbial growth and enzymatic activity and extending the drug's expiration date.
- Simplified logistics. Lyophilized formulations often do not require cold chain transportation, which simplifies logistics and reduces costs, especially in areas lacking cold chain facilities.
- Retention of activity. Lyophilized formulations maintain the structure and function of proteins, peptides, and other bioactive molecules, preventing inactivation during storage and transportation.
- Reduced volume. The lyophilization process significantly reduces the volume of the drug formulation, making it easier to store and transport.
Explore Our Lyophilized Gene Therapy Formulation Development
We focus here on the development of lyophilized adeno-associated virus products. We provide comprehensive technical support and solutions for lyophilized adeno-associated viruses products, and we play an important role in evaluating the formulation excipients required to develop stable lyophilized adeno-associated viruses products and optimizing the concentration and ratio of these excipients to achieve short-term solution stability at higher temperatures as well as long-term stability under refrigeration conditions after lyophilization.
Evaluation of physicochemical properties
The feasibility of lyophilized lentiviral vectors was investigated by using the pre-prescription of lyophilized protective agents, and the lyophilized lentiviral vectors with good appearance, physical and chemical properties, such as appearance, color, and resilience, were prepared.
Determination of biological titer and recovery
The biological titer of lentiviral vectors was investigated by flow cytometry (FACS) and real-time fluorescence quantitative nucleic acid amplification detection system (QPCR).
Selection of lyophilization protectants
Lyophilization protective agents are generally compounds such as sugars, amino acids, proteins and polymers. The selection of lyophilization protectants requires multiple considerations, including the characteristics of the drug molecule, the requirements of the lyophilization process, and the needs of clinical applications.
Determination of residual moisture content
We determined the optimal lyophilization agent prescription by screening and optimizing the prescription of lentiviral vector lyophilization agent, and tested the residual moisture content of the prepared lentiviral vector lyophilized formulations. The residual moisture content of the lyophilized lentiviral vector formulation was determined by Karl Fischer hydrometry.
Stability study
The qualified lyophilized products should be loose and porous, with uniform color and fine texture. In this section, we focus on the stability of the lyophilized preparation, mainly through the high temperature, storage time, repeated freezing and thawing and other factors affecting the stability of the study, the lyophilized preparation of the apparent texture and other conditions to observe.
 Fig.1 Role of lyophilized gene therapy formulation. (CD Formulation)
Fig.1 Role of lyophilized gene therapy formulation. (CD Formulation)
Applications of Our Lyophilized Gene Therapy Formulation Development
- Production of complex formulations. Lyophilization technology is used to produce complex biologics, including proteins, peptides, and other biomolecules, and lyophilized formulations help maintain the stability and activity of these molecules.
- Long-term storage and supply chain management. Lyophilized formulations can be stored stably at room temperature, reducing reliance on cold chain transportation and simplifying supply chain management.
- Global health programs. The stability and convenience of lyophilized formulations are especially important when drugs need to be transported to areas lacking cold chain facilities, helping to achieve global health program coverage.
- Commercial production. As gene therapy products are gradually entering the market, the development of lyophilized formulations is important to enable commercial production to meet the needs of large-scale production.
Our Platforms for Lyophilized Gene Therapy Formulation Development
| Technologies & Platforms |
Content Description |
| Flow Cytometry (FACS) |
A technique used for rapid, high-throughput analysis and sequencing of cells, microorganisms, small particles, and more. It provides information about cell properties by measuring their physical and chemical characteristics and has an important role in the development of gene therapy formulations. |
| Quantitative Polymerase Chain Reaction (qPCR) |
A highly sensitive and precise molecular biology technique used to quantify the amount of a specific DNA or RNA sequence. In lyophilized formulation development, qPCR can play an important role. |
| Freeze-drying technology platform |
This process involves the steps of pre-freezing, primary drying (sublimation drying) and secondary drying (desorption drying) to remove water from the drug and keep it alive. |
Highlights of Our Lyophilized Gene Therapy Formulation Development
- Accuracy. We will calibrate regularly in the project development, etc., reagents standardized procurement, with complete calibration and QC.
- Security and confidentiality. We have a strict hierarchical management mechanism of data rights to ensure data security.
- Personnel qualification. All of our testing personnel-related training and qualification materials are permanently stored, personnel have regular training, and operation has the appropriate training and authorization.
- Record keeping. After the completion of the project, separate paper and electronic files filed records.
Published Data
Technology: adeno-associated viruses lyophilized formulation development
Journal: Curr Pharm Des
IF: 3.1
Published: 2010
This study developed a lyophilized (freeze-dried) formulation of adeno-associated virus. The authors determined that adeno-associated virus could be lyophilized in sucrose and citrate formulations. An optimal residual moisture range (1-3%) was found to be critical for maintaining adeno-associated virus stability. Glycerol was found to protect the adeno-associated virus from overdrying by preventing capsid damage and genomic DNA release. Lyophilized formulations were identified to maintain potency at 2-8°C for 24 months, demonstrating the feasibility of dried formulations for adeno-associated virus gene therapy.
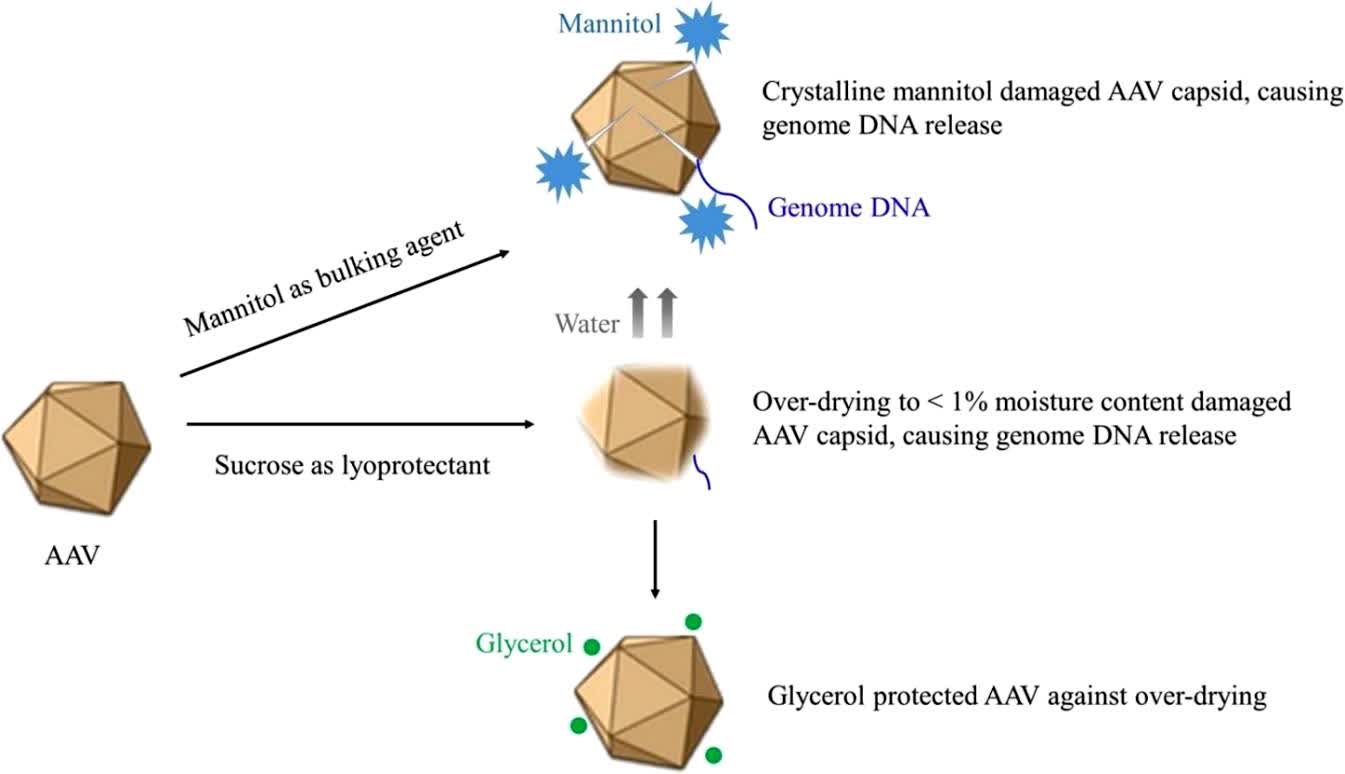 Fig.2 Adeno-associated virus lyophilized formulation development. (Zhang YZ, et al., 2010)
Fig.2 Adeno-associated virus lyophilized formulation development. (Zhang YZ, et al., 2010)
CD Formulation provides a comprehensive solution for the development of lyophilized gene therapy formulations by taking into account various factors such as the stability of the biologically active substance, the optimization of the formulation process, the assurance of sterility, and the long-term storage and transportation of the product. If you are interested in us, please feel free to contact us.
References
- Zhang YZ, et al. Development of a stable lyophilized adeno-associated virus gene therapy formulation. Int J Pharm. 2021, 606:120912.

 Fig.1 Role of lyophilized gene therapy formulation. (CD Formulation)
Fig.1 Role of lyophilized gene therapy formulation. (CD Formulation) Fig.2 Adeno-associated virus lyophilized formulation development. (Zhang YZ, et al., 2010)
Fig.2 Adeno-associated virus lyophilized formulation development. (Zhang YZ, et al., 2010)