Lipid Nanoparticle Development
InquiryCD Formulation is a leading formulation development company focused on advanced nucleic acid therapeutics. We have extensive expertise in the rational design and optimization of lipid nanoparticles (LNPs) for the targeted delivery of a wide range of nucleic acid cargoes. Our team of formulation scientists can customize lipid compositions and engineer nanoparticles with tailored properties.
What are LNPs?
LNPs are spherical vesicles composed of physiological lipids that can encapsulate nucleic acids like RNA, DNA and oligonucleotides. When formulated below 100nm, these nanoparticles can leverage endogenous lipid uptake pathways to ferry cargo into cells. Nucleic acid therapeutics can be delivered using LNPs because of their biodegradable nature and tunable surface properties.
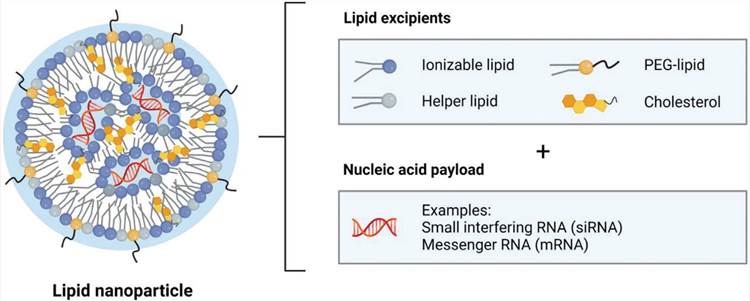 Structure of LNPs loaded with nucleic acids (Ferhan A.R.; et al., 2022)
Structure of LNPs loaded with nucleic acids (Ferhan A.R.; et al., 2022)
Our Lipid Customization Services
Our medicinal chemists design and synthesize novel cationic, ionizable and PEGylated lipids to improve transfection efficiency. Through customization, we optimize parameters like tail length, saturation, branching, and the incorporation of biodegradable elements, enhancing the performance and functionality of LNPs.
How Do We Develop LNPs?
Our formulation experts select the appropriate lipids and solvents for nucleic acid drugs, systematically evaluating and controlling interdependent variables such as lipid composition, nucleic acid ratios and manufacturing parameters.
We leverage microfluidic technology where organic solutions of lipids and aqueous solutions of nucleic acids are precision-mixed within micron-scale channels via laminar flow. Microfeatures induce controlled mixing at the fluid-fluid interface.
During rapid solvent shift, lipids spontaneously assemble around encapsulated nucleic acids to form monodisperse nanoparticles within milliseconds as they exit the device.
Systematic variation of fluid velocities and ratios tunes critical product attributes such as nanoparticle size, drug release, and transfection efficiency. pH-responsive lipids help deliver payloads intracellularly.
Our LNP Characterization Services
|
Particle Characterization |
- We employ techniques such as DLS, TEM, and NTA to determine the particle size distribution, morphology, and structural properties of LNPs.
|
| Zeta Potential Analysis |
- The surface charge of LNPs is measured using electrophoretic light scattering techniques.
|
| Encapsulation Assessment |
- Quantitative methods like HPLC and UV-Vis spectroscopy are utilized to quantify the encapsulation efficiency and loading capacity of payloads within LNPs.
|
| Cryogenic TEM Imaging |
- Cryo-TEM reveals the high-resolution internal morphology of fully hydrated nanoparticles.
|
| Release Kinetic Analysis |
- In vitro assays using dialysis or centrifugal filtration continuously profile cargo release from LNPs over time.
|
| Stability-Indicating Studies |
- Forced degradation studies coupled with analytical methods verify the stability of LNPs under various storage conditions.
|
| Material Characterization |
- Spectroscopic techniques provide insights into components, molecular interactions and crystallinity within LNPs.
|
| Sterility and Endotoxin Testing |
- We can detect bacterial and endotoxin residues in samples to ensure product quality and safety.
|
Applications of Our LNP Development Services
We design LNP delivery systems for novel mRNA vaccines against infectious diseases and cancer.
Our expertise in nucleic acid encapsulation supports the development of RNAi drugs for genetic disorders and cardio-metabolic diseases.
We develop LNPs to efficiently deliver CRISPR/Cas9 and donor DNA for in vivo gene editing applications.
- Oligonucleotide Therapeutics
Our platforms are well-suited to antisense oligonucleotides, siRNAs and microRNAs to modulate gene expression.
Highlights of Our LNP Development Services
- Our medicinal chemists can optimize lipid compositions for targetable delivery platforms.
- Our LNPs are versatile carriers for various therapeutic payloads, including nucleic acids and other drugs.
- We offer integrated capabilities across chemistry, physics, engineering and biology to fully optimize LNP formulations.
- Our customized approaches address specific cargo properties, disease targets and administration routes.
- Our platform technologies have advanced over a dozen nucleic acid programs through various stages.
If you would like a customized quote for your LNP development program, please contact us!
References
- Ferhan A.R.; et al. Lipid nanoparticle technologies for nucleic acid delivery: A nanoarchitectonics perspective. Adv Funct Mater. 2022, 32(37): 2203669.
- Evers M.J.W.; et al. State‐of‐the‐art design and rapid‐mixing production techniques of lipid nanoparticles for nucleic acid delivery. Small Methods, 2018, 2(9): 1700375.
- Cárdenas M.; et al. Review of structural design guiding the development of lipid nanoparticles for nucleic acid delivery. Curr Opin Colloid Interface Sci. 2023: 101705.
Related Services


 Structure of LNPs loaded with nucleic acids (Ferhan A.R.; et al., 2022)
Structure of LNPs loaded with nucleic acids (Ferhan A.R.; et al., 2022)