For over 15 years, CD Formulation has provided world-class analytical services to pharmaceutical companies in the animal health sector. Our pharmacokinetics studies characterize the time course of drug absorption, distribution, metabolism and excretion using biological fluids such as plasma, urine and feces.
What is the Pharmacokinetic?
Pharmacokinetics of veterinary drugs describes the bodily absorption, distribution, metabolism and excretion of these compounds.
- Absorption studies examine how quickly and completely the drug is taken up by the body through various routes.
- Distribution research investigates the movement and concentration of drug molecules to target tissues.
- Metabolism experiments explore the biochemical modifications drugs undergo, and their active versus inactive breakdown products.
- Elimination tests track the removal of the original drug and its metabolites from the body over time.
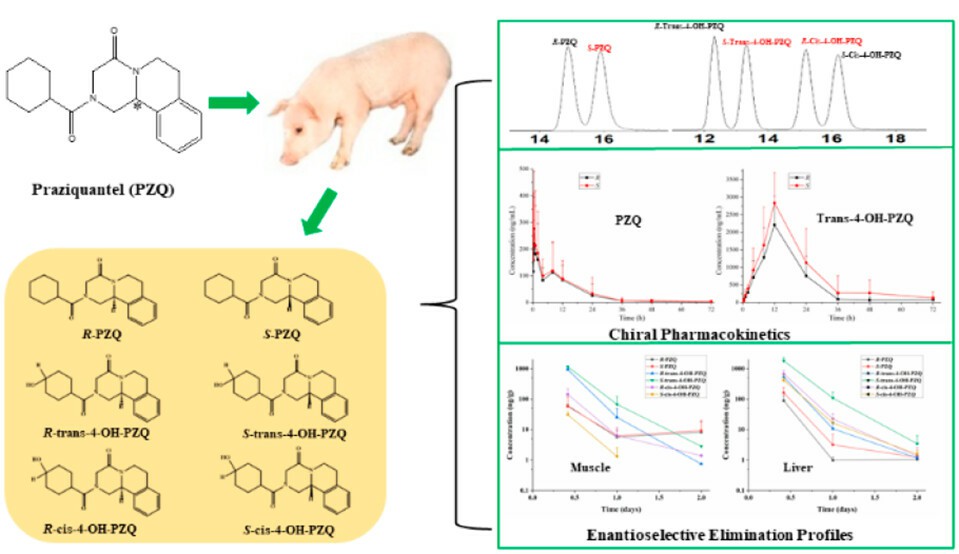 Pharmacokinetics of praziquantel and its metabolites in swine (Zhang M.; et al. 2023)
Pharmacokinetics of praziquantel and its metabolites in swine (Zhang M.; et al. 2023)
What Can We Do for Veterinary Drug Pharmacokinetics Research?
|
Absorption Studies |
We determine the rate and extent of drug absorption through intensive plasma sampling after administering doses via various routes such as oral, subcutaneous, intramuscular, or intravenous. Our bioanalytical techniques identify the parent drug and metabolites present in the plasma samples. |
| Distribution Studies |
To investigate drug distribution patterns, we employ microdialysis probes implanted in tissues to collect interstitial fluid over time for analysis. Additionally, we utilize radiolabeled drugs to examine distribution by measuring tissue counts or utilizing imaging modalities like PET scans. |
| Metabolism Studies |
Our phenotyping studies involve inhibition assays to clarify the role of specific CYP isozymes in drug metabolism. Through MetID research, we identify metabolites using advanced techniques such as mass spectrometry (MS) and nuclear magnetic resonance (NMR) following incubation with hepatocytes, S9 fractions, or recombinant enzymes. |
| Excretion Studies |
We conduct comprehensive excretion studies, including total mass recovery tests that involve collecting feces and urine in metabolic cages. Additionally, we assess biliary excretion using cannulated models, quantifying the amounts of drugs and metabolites eliminated over defined intervals. |
| Protein Binding |
To determine the active drug levels, we employ equilibrium dialysis coupled with analytical methods to differentiate between bound and free fractions of drugs in plasma, providing insights into protein binding characteristics. |
| Modeling Services |
Our team utilizes non-compartmental and modeling approaches to derive essential pharmacokinetic parameters like volume of distribution, clearance, and half-life from concentration-time profiles, enabling a comprehensive understanding of drug kinetics. |
| Bioequivalence Studies |
We conduct standardized study designs that generate extensive pharmacokinetic parameters to determine the substitutability of different dosage forms, ensuring bioequivalence. |
Our Platforms for Veterinary Pharmacokinetics Research
| In Vivo |
- Purpose-bred laboratory animals (mice, rats, dogs, pigs, monkeys etc.)
- Dedicated animal housing and surgical facilities
- Sampling capabilities (blood, tissues, excreta)
- Analytical instrumentation for bioanalysis (LC-MS/MS, GC-MS, immunoassays)
- Pharmacokinetic modeling software
|
In Vitro |
- Tissue/cell culture laboratories
- Hepatocyte isolation and culture
- Subcellular fractions (microsomes, S9)
- Metabolites identification capabilities
- High resolution mass spectrometry
- NMR
- Cytrochrome P450 inhibition assays
- Protein binding assays
|
Applications of Our Veterinary Pharmacokinetics Research
By studying the pharmacokinetics of veterinary drugs, we provide insights into optimal dosing regimens, including dosing frequency and dosage adjustments based on factors such as animal species, age, and health condition. This helps veterinarians administer medications effectively and maximize therapeutic outcomes.
- Drug Interaction Assessment
We investigate drug-drug interactions in veterinary patients to determine potential interactions between multiple medications. By analyzing the pharmacokinetic profiles of drugs, we can identify drug interactions that may affect efficacy, safety, or dosing requirements, allowing veterinarians to make informed decisions regarding drug combinations.
- Species-Specific Variability
Veterinary pharmacokinetic studies consider species-specific differences in drug metabolism and disposition. Understanding these variations helps tailor drug therapies for different animal species, accounting for factors such as metabolism rates, bioavailability, and elimination pathways.
Why Choose Our Pharmacokinetic Services?
- Comprehensive profiling of full ADME characterization and PK parameters.
- Expertise in biopharmaceutics, PK modeling/analysis, and PK-PD correlations for optimized profiling.
- Dedicated team of veterinary scientists, pharmacologists and bioanalytical experts.
- Advanced bioanalytical capabilities using validated LC-MS/MS and immunoassay methods.
- Validated preclinical animal models to optimally represent target veterinary species.
CD Formulation understands the unique needs of the veterinary pharmaceutical industry. We have the expertise to conduct thorough ADME profiling and PK analyses across multiple relevant species. If you would like to discuss how our fully integrated pharmacokinetics platform can benefit your next project, please contact us!
Reference
- Zhang M.; et al. Stereoselective pharmacokinetics and residue depletion of praziquantel and its metabolites, 4-hydroxypraziquantel enantiomers, in swine. J Agric Food Chem. 2023, 71(31):12061-12069.


 Pharmacokinetics of praziquantel and its metabolites in swine (Zhang M.; et al. 2023)
Pharmacokinetics of praziquantel and its metabolites in swine (Zhang M.; et al. 2023)