In Vitro Penetration Testing of Transdermal Formulation
Inquiry
The in vitro permeation test (IVPT) is a crucial research tool used for evaluating skin delivery preparations. It simulates the transdermal process of drugs under physiological conditions to measure the rate and extent of active ingredients reaching the site of action. This helps in assessing the quality of drugs and the effectiveness of clinical treatment. CD Formulation specializes in in vitro and ex vivo models for conducting quality analysis of transdermal formulations and provides solutions and services to the pharmaceutical industry.
About In Vitro Penetration Testing
Compared with traditional in vivo tests, IVPT can explore skin permeability differences in the early absorption stage by accurately measuring the distribution, penetration amount and penetration rate of active ingredients of transdermal formulation in each skin layer. The method avoids the use of live animals and allows for repeated testing of multiple identical or different active ingredients and is particularly suitable for transdermal drug screening tests comparing different transdermal formulations. In addition, IVPT studies are also crucial for evaluating the bioequivalence (BE) of topical products. Combined with the results of in vitro release testing (IVRT), we can provide important evidence for isotropic evaluation of transdermal formulation. It is these advantages that IVPT has a wide range of applications but is not limited to: Help screen early compounds, Formulation development of transdermal formulation, Optimization and characterization of properties of transdermal formulation, BE and bioavailability assessment, Safety assessment of drugs.
What Can We Offer
We can help you develop IVPT methods suitable to support the bioequivalence demonstration of topical preparations. Relevant parameters include:
| Technologies |
Description |
| Skin type selection |
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) recommend using isolated human skin for testing. At CD Formulation, our team of scientists will select the best skin tissue type based on the clinical application site and characteristics of your product to ensure that the test results are representative. This may include the skin of animal origin such as pigs, guinea pigs, rabbits, mice, etc., as well as the skin of human origin. |
| Temperature and stirring time selection |
Instrumentation should ensure consistent temperature control throughout the experiment. In CD Formulation, the mixing speeds of different instruments are different. We will control the mixing or stirring speed so that the interface leakage state can be maintained and the skin surface temperature can be stabilized at (32±1)°C. |
| Receiving media selection |
In CD Formulation, we determine the selection of receiving media based on the solubility and stability of the drug in the receiving medium to ensure the accuracy of test results. |
| Sampling time point selection |
During the development phase of the IVPT method, we set the duration of the test and sampling time points based on the properties of the target compound and the characteristics of the formulation. The number of sampling time points should cover the entire process of absorption change, i.e. capture the maximum absorption rate and the subsequent decrease in absorption rate. |
| Dosage and method of administration selection |
At CD Formulation, our in vitro dermal delivery is available in two dosage forms, limited dose and unlimited dose. According to the characteristics of the product, samples can be selected in two ways, weight and volume. |
Through a series of exploratory studies, the selected parameters of the IVPT method are proved to be reasonable. Our main research methods include the vertical diffusion cell method and the flow diffusion cell method.
- The flow-type diffusion cell has a continuous flow of receiving fluid to simulate the flow of blood in the body, but due to the introduction of new parameters, more pre-tests are needed to determine the relevant test parameters.
- The vertical static diffusion cell has a fixed volume receiving chamber, and the receiving liquid is continuously stirred during the test, which is our most commonly used research method.
Our Workflow for In Vitro Penetration Testing
Our in vitro penetration testing process involves the following steps: First, select an appropriate in vitro penetration model. Then, prepare the experimental materials and select the skin according to the experimental requirements. Next, assemble the experimental device, ensuring that all components are correctly installed. Set the experimental conditions and collect samples at specified time intervals. Finally, analyze the samples and process the data.
 Fig.1 Flow chart of in vitro penetration testing (CD Formulation)
Fig.1 Flow chart of in vitro penetration testing (CD Formulation)
Our In Vitro Penetration Testing Technology Platform
| Technologies & Platforms |
Description |
| Precise quantitative analysis instruments |
We are equipped with advanced analytical instruments, such as high performance liquid chromatography (HPLC), gas chromatography (GC), mass spectrometry (MS), etc., which can conduct precise quantitative analysis of drugs or chemical substances during the penetration process. This high-precision analysis ensures the accuracy and reliability of experimental results. |
| Flexible experimental conditions |
We have different skin models (such as human skin, animal skin or synthetic membranes) that can be adjusted and optimized according to different testing needs to adapt to the testing needs of different products. The animal skins we stock include pig, rabbit, rat, mouse, etc. The skin of these animals is structurally similar to human skin, especially pig skin, whose permeability is closer to human skin, so it is often used in transdermal absorption research. In addition, there are differences in the permeability of the skin of different animals. For example, the skin permeability of pigs and monkeys is similar to that of human skin, while the skin permeability of rabbits, rats, and guinea pigs is greater than that of human skin. |
Highlights of Our In Vitro Penetration Testing Services
- Cost saving: Compared with animal experiments and human experiments, the cost of CD Formulation`s IVPT experiments is relatively low. There is no need for large amounts of animal or human samples, nor complex ethical approval and regulatory procedures, so research and development costs and time can be greatly saved.
- Highly simulate physiological conditions: We can highly simulate the physiological conditions of human skin or biofilm, such as pH value, temperature, humidity, etc., thereby more accurately simulating the penetration process of drugs or chemical substances in the body.
- High flexibility: Our IVPT has high flexibility and can adapt to the evaluation needs of different drugs and preparations. Different sources of ex vivo skin, delivery methods and analysis methods can be selected according to needs to provide personalized solutions for drug research and development.
Published Data
Technology: Methods to In Vitro Penetration Testing
Journal: Scientia. Pharmaceutica
IF: 2.5
Published: 2019
Results: In this research, the most well-known and recent methods for assessing in vitro skin penetration of drugs are evaluated, the various experimental models used in transdermal studies are presented, and the advantages and disadvantages of different methods are compared. These models offer the possibility of rapid screening and faster product optimization. The selection of the most appropriate in vitro model should be based on availability, ease of use, cost, and respective limitations.
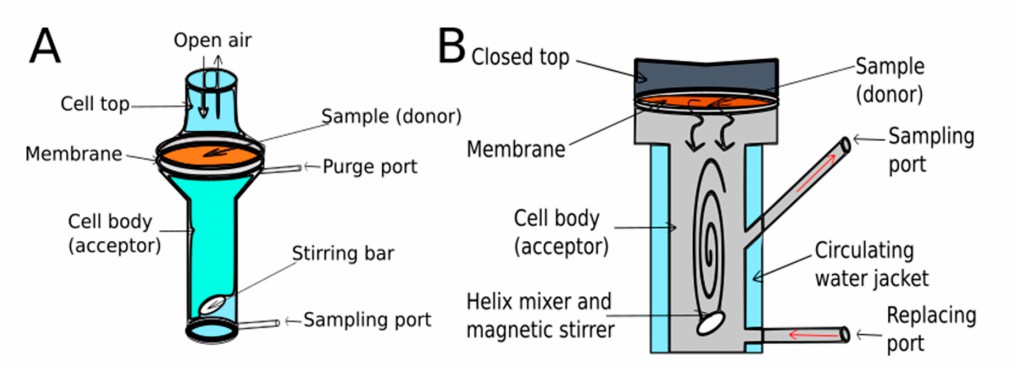 Fig.2 Open (A) and closed (B) vertical Franz diffusion cells (Stella Zsikó, et al., 2019)
Fig.2 Open (A) and closed (B) vertical Franz diffusion cells (Stella Zsikó, et al., 2019)
The percutaneous absorption of drugs is a multi-factor and multi-step process, and the results of in vitro percutaneous penetration are also affected by many factors. At CD Formulation, our professional team is committed to providing reliable and comprehensive in vitro penetration testing services to ensure the accuracy of experiments and protect your drug development and launch. If you have any needs, please feel free to contact us and our colleagues will reply to you within three working days.
References
- Stella Zsikó , Erzsébet Csányi, et al. Methods to Evaluate Skin Penetration In Vitro. Scientia. Pharmaceutica. 2019, 87(3), 19.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Flow chart of in vitro penetration testing (CD Formulation)
Fig.1 Flow chart of in vitro penetration testing (CD Formulation) Fig.2 Open (A) and closed (B) vertical Franz diffusion cells (Stella Zsikó, et al., 2019)
Fig.2 Open (A) and closed (B) vertical Franz diffusion cells (Stella Zsikó, et al., 2019)
