Sublingual Thin Film Development
Inquiry

The sublingual thin film delivery system refers to the placement of a thin oral strip under the tongue, which is immediately moistened by saliva, and the film rapidly hydrates and adheres to the site of application. It then rapidly disintegrates and dissolves to release the medication for absorption by the oral mucosa and deliver its efficacy. With professional formulation knowledge and service flexibility, CD Formulation provides efficient and reliable formulations of sublingual thin film development services.
Why Develop Sublingual Thin Film?
The sublingual mucosa lacks a cuticle and possesses high permeability and abundant capillaries. The sublingual gland, situated within this mucosa, secretes and accumulates saliva, facilitating rapid drug dissolution. Research indicates that drug absorption via the gastrointestinal tract typically takes 10 to 20 minutes to elicit effects, whereas sublingual administration achieves this in just 30 to 60 seconds, making it 10 to 20 times faster than oral intake. Consequently, many emergency medications are administered sublingually.
In comparison to conventional dosage forms, the sublingual oral film drug delivery system offers distinct advantages. Particularly for low doses and small molecules, sublingual oral thin films present a feasible alternative to injections. Various forms of active pharmaceutical ingredients (APIs), such as micropowder, granular, salt, and free base forms, can be formulated into sublingual thin films. This enables precise dosage delivery by controlling the size and thickness of the film. Moreover, sublingual thin films facilitate direct drug absorption into systemic circulation through mucosal tissue, circumventing first-pass metabolism and enhancing bioavailability. Emerging sublingual thin film drug delivery systems address diverse medication and patient needs, including life-cycle management improvements and easier administration for pediatric, geriatric, and psychiatric patients with dysphagia.
Our Sublingual Thin Film Formulation Development Services
As a global leader in oral thin film development, our team of experts is dedicated to the development of sublingual thin film based on an advanced technology platform and professional drug development experience, from the formulation design of sublingual thin film, formula process evaluation, quality control to commercial production, we will provide you with a one-stop solution for the whole process.
Formulation Design
Our team of experts will often carefully consider the rationality and necessity of the dosage form based on your therapeutic purpose and properties of the APIs and combine whether it can fully prove its clinical advantages through reasonable design studies (pharmaceutical and clinical aspects), provide you with standardized formulation design information to determine the effectiveness, quality controllability and dosage form selection rationality of the final product.
Formula Process Evaluation
In the formula process development of sublingual thin film, special attention needs to be paid to the basic properties of the APIs, such as crystal form, particle size, solubility, etc. Our team of experts will further evaluate the type and dosage selection of APIs and excipient materials according to the quality target product profile (QTPP) and critical quality attributes (CQA) of the preparation and combine the compatibility testing of APIs and excipient materials to confirm the rationality of the formula.
Quality Control
Our experienced technicians carry out comprehensive quality control, including aspects of appearance, physical and chemical properties, biological characteristics etc.
Commercial Production
We have very rich experience in pilot transfer of sublingual thin film, our technical teams achieve a reasonable transition from laboratory scale, pilot scale to commercial specifications through key process steps and parameters, process control, intermediate control, etc.
Our Platforms for Sublingual Thin Film Formulation Development
| Technologies & Platforms |
Specifics Contents |
| Formulation Design Platform |
Evaluate the feasibility of dosage forms and design formulas based on the properties of raw materials and therapeutic purposes. |
| Preparation Technology Platform |
We have a solvent casting technology platform, solvent casting technology is a common preparation technology for sublingual thin film, which comprises of casting a dope from a casting die onto a casting support, drying the cast dope on the casting support form film. |
| Analysis and Characterization Platform |
We can offer multiple analysis methods to characterize the properties of APIs, including crystal shape, particle size, solubility, etc., and study the compatibility of APIs and excipient. |
Our Advantages in Sublingual Thin Film Development
- Efficient Development Process: We are familiar with the development process and quality control indicators of sublingual thin film and can quickly identify the difficulties in the development process and speed up the development of sublingual thin film.
- Strict Quality Standards : We adhere to the highest quality standards to ensure that the sublingual thin film developed complies with all laws and regulations.
- Rich Development Experience : We have many years of research and rich experience in the formulation development of sublingual thin films.
- One-Stop Solutions : We provide one-stop services for sublingual thin film, which can help customers save research time to a great extent.
Published Data
Technology: Solvent casting technique
Journal: Journal of Drug Delivery Science and Technology
IF: 5
Published: 2017
Results: In this research, FSM was successfully incorporated into mucoadhesive films which could be delivered through the sublingual route. HEC was used as a mucoadhesive polymer for sublingual delivery along with various penetration enhancers resulting in films with satisfactory physico-mechanical properties, good mucoadhesive strength, fast drug release and maximum permeation without causing any damage to porcine sublingual mucosa. Finally, TPGS was loaded in film to further improve the drug permeability.
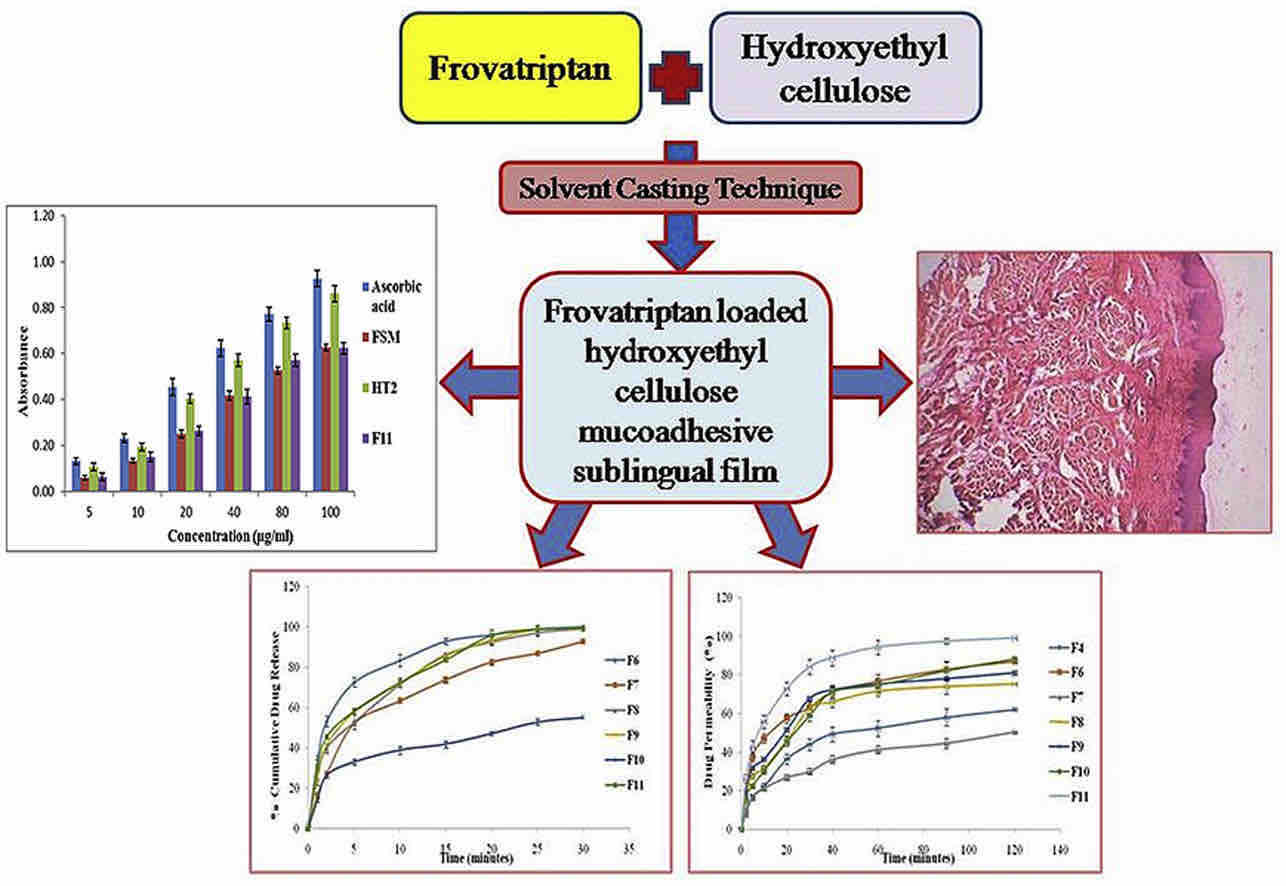 Fig.1 Formulation and design of frovatriptan loaded hydroxyethyl cellulose mucoadhesive sublingual film. (Harmanpreet Singh, et al., 2017)
Fig.1 Formulation and design of frovatriptan loaded hydroxyethyl cellulose mucoadhesive sublingual film. (Harmanpreet Singh, et al., 2017)
CD Formulation is dedicated to the development of sublingual thin film and always pays attention to the frontier development of oral thin films. We continue to overcome difficulties, and explore innovation, relying on our advanced oral thin film technology platform, to provide effective services for the development of sublingual thin film. If you have a requirement about our sublingual thin film development services, please contact us by phone or email, our colleagues will reply to you within three working days.
References
- Harmanpreet Singh, Jasjeet Kaur Narang, et al. TPGS stabilized sublingual films of frovatriptan for the management of menstrual migraine: Formulation, design and antioxidant activity. Journals & Books. 2017, Volume(41) : 144-156.
- Dhendi Sandhya, P. Jyothi. Formulation and In Vitro Evaluation of Ondansetron Oral Thin Films. Human Journals. 2023, Vol(41): 301-304.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services





