Solid Dispersion Extrusion Method for Oral Thin Film Preparation
Inquiry
As more and more attempts are made to manufacture drug thin films, many technologies are introduced or customized to achieve high process reproducibility and optimized quality in drug delivery applications. The solid dispersion extrusion method is a new manufacturing technique. As the name suggests, the process involves the dispersion of one or more active pharmaceutical ingredients (APIs) in a solid state in an inert carrier using methods like HME. The immiscible components are extruded with the drug, which are further converted into solid dispersions. These dispersions are shaped into films using dies. As a leader in innovative drug development, CD Formulation dares to experiment and has established a solid dispersion extrusion technology platform to assist in the development of oral thin film drug delivery systems.
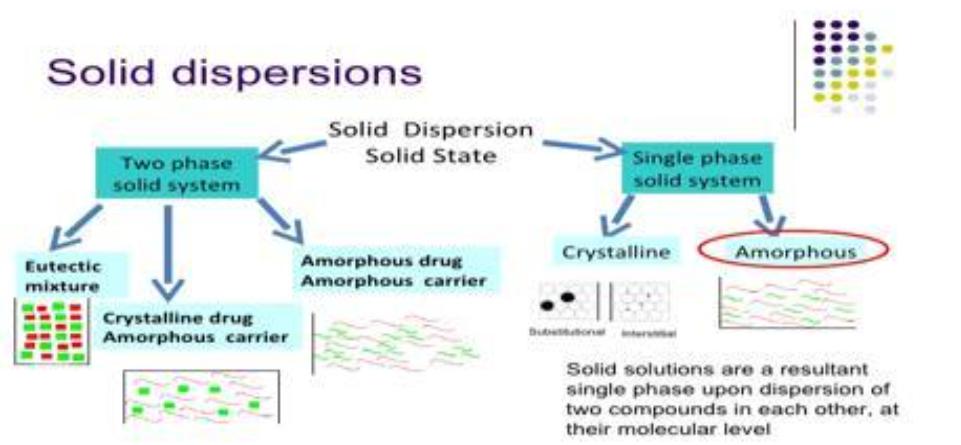 Fig.1 Solid Dispersion Extrusion Method. (Pawar Rajat, et al., 2016)
Fig.1 Solid Dispersion Extrusion Method. (Pawar Rajat, et al., 2016)
The Advantages of the Solid Dispersion Extrusion Method for Oral Thin Film Preparation
- Improve the solubility of insoluble drugs
- Increase disintegration and dissolution and reduce the frequency of administration
- Enhance drug stability
- Mask the bitter taste of drugs
- Gain the desired release profile
Solid Dispersion Extrusion Process for Oral Thin Film Preparation
- Preparation Process of Solid Dispersion Extrusion
Solid dispersion extrusion is a method for manufacturing oral thin films that uses the solid dispersion method.
When one or more active chemicals are dispersed in an inert carrier in a solid form while amorphous hydrophilic polymers are present, this is referred to as solid dispersion. In this process, medications are dissolved in suitable solvents before being added to the polyethylene glycol melt at a temperature below 70 °C. Finally, using dies, solid dispersion is molded into the thin films.
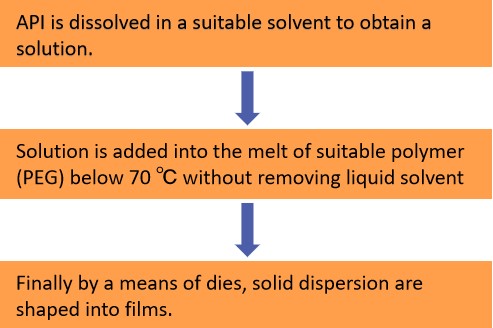 Fig.2 Workflow of Solid Dispersion Extrusion Method for Oral Thin Film Preparation. (CD Formulation)
Fig.2 Workflow of Solid Dispersion Extrusion Method for Oral Thin Film Preparation. (CD Formulation)
- Characterization Method of Solid Dispersion Extrusion Technology for Oral Thin Film Preparation
In the process of preparing the oral soluble film by solid dispersion technology, it is necessary to characterize the amorphous solid dispersion after obtaining it to determine the dispersion state of the drug in the carrier. In addition, the solid dispersion is in a high-energy unstable state, and the drug molecules may spontaneously aggregate into crystal nuclei, and the microcrystals gradually grow into grains, and eventually transform into stable crystals. Therefore, we will pay close attention to the change of its state and conduct a comprehensive evaluation from the aspects of solubility, dissolution rate, stability, etc., to provide more data support for subsequent research and development.
Our Technologies & Platform
When preparing oral thin films using solid dispersion extrusion techniques, the dispersion needs to be characterized, and our characterization capabilities include but are not limited to:
| Technologies & Platform |
Description |
| X-ray Diffraction (XRPD) Platform |
The X-ray diffraction is like a person's fingerprint, which has a specific property. The diffraction peak of the crystalline substance is sharp, while the drug in the solid dispersion exists in an amorphous state, and its crystallization diffraction peak will disappear, sometimes appearing as a steamed bun.
|
| Thermal Analysis Platform |
Thermal analysis shows that the physical properties of the measured system change with temperature, and Differential Scanning Calorimetry (DSC) is the most common method. The thermal signal on the DSC curve can reflect the melting, crystallization, phase transformation and decomposition of the sample.
|
| Polarizing Microscope Platform |
The polarizing microscope can intuitively observe and distinguish between crystal and amorphous structure, through whether the sample has birefringence optical properties to identify whether it is crystalline state, but also can quickly understand the shape and particle size of the sample.
|
| Scanning Microscope Platform |
Scanning electron microscopy has higher resolution and can reflect the morphology of samples and detect the surface morphology of solid dispersion more directly.
|
| Infrared Spectroscopy, IR Platform |
When the drug does not interact with the carrier, the infrared profile of the solid dispersion is usually the same as that of the physical mixture. When the drug interacts with the carrier, the infrared absorption peak displacement or peak intensity will change, so as to achieve the purpose of identification.
|
| Raman Spectroscopy, RM Platform |
Raman spectrum is a scattering spectrum, the general infrared absorption is not obvious non-polar groups Raman spectrum absorption is obvious. In terms of structure analysis and phase identification, it can complement infrared spectroscopy to obtain more comprehensive results.
|
| Solid-State Nuclear Magnetic Resonance, SSNMR Platform |
Solid-state nuclear magnetic resonance (NMR) is a method for qualitative identification and quantitative detection of crystalline and amorphous forms. It can help to understand the motility of molecules in samples and the crystallization rate of amorphous drugs, and can also be used in the study of the interaction force between drugs and polymers.
|
Why Choose Us for Preparing Oral Thin Films with the Solid Dispersion Extrusion Method?
- Innovative Manufacturing: We employ innovative solid dispersion extrusion manufacturing processes that are fully scalable and efficiently meet commercial demands.
- Rich Experience: With many years of experience in solid dispersion extrusion production of oral thin film, we can quickly help customers solve potential problems in this preparation technology and accelerate the development of oral thin film.
- Comprehensiveness: We possess an advanced analysis and characterization technology platform that provides comprehensive characterization services for the dispersion system during the preparation of oral thin film by solid dispersion extrusion technology, laying the foundation for the production of high-quality oral thin film.
Published Data
Technology: Solid Dispersion method
Journal: Macromolecular Research
IF: 2.4
Published: 2020
Results: In this study, we chose to fabricate oral thin film with our optimized solid dispersion method. Oral thin film allows for both the rapid and efficient dissolution within the oral cavity and the protection of the active ingredient (i.e., OM) from rapid degradation under acidic conditions.
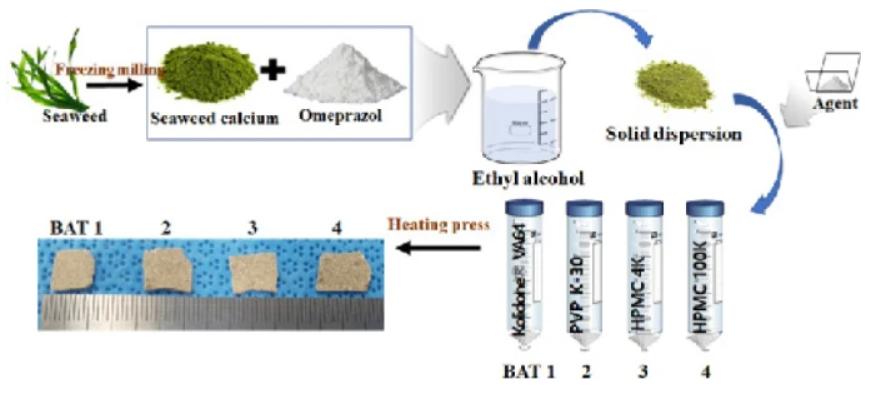 Fig.3 Omeprazole oral soluble film was prepared by dispersion method. (Won Kyung Kim, et al., 2020)
Fig.3 Omeprazole oral soluble film was prepared by dispersion method. (Won Kyung Kim, et al., 2020)
CD Formulation can help you solve any problems that arise in the solid dispersion extrusion method to prepare the oral thin film. If you have a requirement about our solid dispersion extrusion method for oral thin film preparation services, please
contact us by phone or email, and our colleagues will reply to you within three working days.
References
- Pawar Rajat, Sharma Ravi, et al. A Review on Mouth Dissolving Film. Journal of Drug Delivery and Therapeutics. 2019, 9(6):206-210.
- Won Kyung Kim, Hun Hwi Cho, et al. Alleviated Side Effects and Improved Efficiency of Omeprazole Using Oral Thin Film: In Vitro Evaluation. Macromolecular Research. 2020, Vol (28):417-424.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services






