Oral Thin Film Preclinical Studies
Inquiry
Oral thin film preclinical studies mainly include formulation screening, preparation technology, test method, quality index, stability, pharmacology, toxicology and pharmacokinetics of experimental animals. The purpose of a preclinical study is to test the safety of drugs. Sometimes, preclinical studies can also provide relevant information about the efficacy of drugs, laying the foundation for human trials of the drugs. The results of preclinical studies can provide sponsors and regulatory authorities, such as the FDA, with sufficient evidence to determine whether the drug can enter clinical trials. CD Formulation is a pharmaceutical research and development service provider that provides preclinical development, integrated services and innovative drug delivery system solutions, providing comprehensive analysis and research, laboratory testing and development services for the entire process from drug discovery to preclinical development for customers in the global oral thin films pharmaceutical industry.
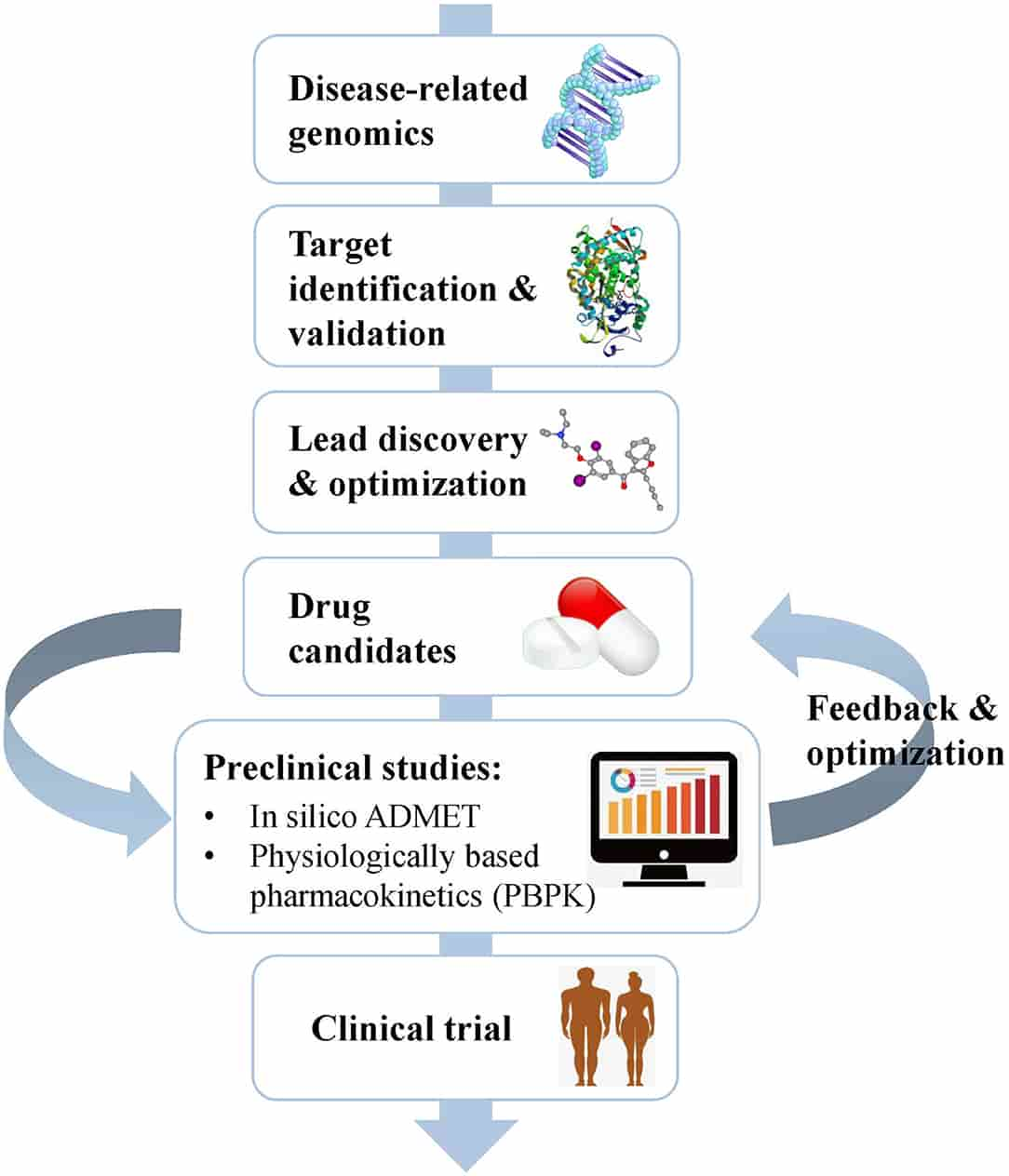 Fig.1 Schematic flow chart summarizing the process of drug discovery and the main content of the preclinical study. (Fengxu Wu, et al., 2020)
Fig.1 Schematic flow chart summarizing the process of drug discovery and the main content of the preclinical study. (Fengxu Wu, et al., 2020)
The Importance of Oral Thin Film Preclinical Studies
Preclinical research plays a crucial role in the oral thin film drugs development process, and its importance is mainly reflected in the following aspects:
- Ensure patient safety:
Through preclinical research, the potential risks of oral thin film drugs can be discovered as early as possible to ensure the safety of patients.
- Improve the efficiency of drug development:
Preclinical research can help screen out unqualified oral thin film drug candidates in advance, reducing the time and cost of research and development process.
- Guide clinical trial design:
The results of preclinical studies can provide data support for clinical trial design and improve the success rate of clinical trials.
- Promote drug research and development process:
Preclinical research is the premise and basis of drug research and development and is of great significance to promoting oral thin film drugs research and development process.
Our Preclinical Studies Services for Oral Thin Films
CD Formulation specializes in providing customizable preclinical trial service solutions for oral dissolved membranes. With more than ten years of R&D technology experience, we have professional knowledge in drug metabolism, pharmacokinetics, pharmacodynamic research, and toxicology. Providing you with high quality data and fast turnaround times to support oral thin film drug development, preclinical studies and clinical studies. Help you select high-value drug candidates for clinical trials. Our oral thin film preclinical research services include but are not limited to:
Literature Research
We provide comprehensive literature research and evaluation, including the name and naming basis of oral thin film drugs, and the purpose and basis of the title.
Pharmaceutical Research
We provide pharmaceutical research for oral thin films, focusing mainly on API technology research, formulation and preparation technology research, confirmation of chemical structure and component research, quality testing, drafting and explanation of standards, sample inspection, excipients, stability testing, and packaging materials and container-related tests.
Pharmacological and Toxicological Research
We offer a comprehensive range of toxicology research services for oral thin film drugs, specializing in preclinical animal toxicology tests necessary for the development of oral thin films. Our services include pharmacodynamic tests, acute toxicity tests, long-term toxicity tests, allergic reactions, hemolytic and local irritation tests, mutagenicity tests, reproductive toxicity tests, carcinogenicity tests, dependence tests, and animal pharmacokinetic tests, among others.
Our Platforms for Oral Thin Film Preclinical Studies
| Technologies & Platform |
Description |
| Computer Simulation Technology Platform |
In vitro and in vivo drug evaluation technologies are currently mature for preclinical applications, but these technologies are costly. Our team of experts-built computer simulation technology platform to predict the relevant properties of drugs in the preclinical stage combines the computer simulation platform with in vivo/in vitro studies to enhance predictability. |
| Animal Experiment Platform |
We have established a perfect animal model library to meet the needs of customers for different types of preclinical research of oral thin film drugs. |
| Data Acquisition Systems Platform |
We have established a variety of data acquisition systems; The whole experiment process can be traced to ensure that the declaration requirements are met. |
Advantages of Our Oral Thin Film Preclinical Studies Services
- Comprehensive Platform:
We have a variety of new drug integrated evaluation technology platforms, which can develop personalized preclinical integrated evaluation research strategies based on the characteristics of different types of oral thin films.
- Perfect Animal Model Library:
After years of experience accumulation and multi-party verification, we have established a perfect animal model library to meet the needs of customers for different types of preclinical research of oral thin film drugs.
- Flexibility:
We provide single or complete set of study date for preclinical safety evaluation of oral thin films.
- International Standard: We have established a variety of data acquisition systems in accordance with international standards to strengthen the standardization and traceability of the research process and ensure that the preclinical study submission meets FDA requirements.
Published Data
Technology: Oral Formulations for Preclinical Studies
Journal: Pharmaceutical Theory and Practice
IF: 1
Published: 2017
Results: This study offers basic principles, general approaches, and practical considerations in developing oral formulations for preclinical studies. It provides an overview of formulation and study requirements for safety evaluation, discusses important physicochemical properties and their impact on formulation design and performance, details preclinical formulation approaches with a focus on enabling technologies for poorly water-soluble compounds, and describes current advancements in understanding of dissolution behaviors of enabling formulation and rational design of in vitro dissolution methods. It also addresses pros and cons of various formulation approaches and selections.
CD Formulation has rich and professional development experience in the research and development of oral thin film drugs, specializing in providing customers with customizable preclinical trial solutions. Fast trial start-up and flexible trial design will help you make faster decisions and reduce your research expenditure. If you have a requirement about oral thin film preclinical studies services, please contact us by phone or email, and our colleagues will reply to you within three working days.
References
- Y. Gao, C. Gesenberg, et al. Chapter 17 - Oral Formulations for Preclinical Studies: Principle, Design, and Development Considerations. Pharmaceutical Theory and Practice. 2017: 455-495.
- Fengxu Wu, Yuquan Zhou, et al. Computational Approaches in Preclinical Studies on Drug Discovery and Development. Medicinal and Pharmaceutical Chemistry. 2020, Vol(8): 455-495.
- Y. Gao, C. Gesenberg, et al. Oral Formulations for Preclinical Studies: Principle, Design, and Development Considerations. Pharmaceutical Theory and Practice. 2017:455-495.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services




